A comprehensive analysis of the efficacy and safety of COVID-19 vaccines
- PMID: 34365034
- PMCID: PMC8342868
- DOI: 10.1016/j.ymthe.2021.08.001
A comprehensive analysis of the efficacy and safety of COVID-19 vaccines
Abstract
The numbers of cases and deaths from coronavirus disease 2019 (COVID-19) are continuously increasing. Many people are concerned about the efficacy and safety of the COVID-19 vaccines. We performed a comprehensive analysis of the published trials of COVID-19 vaccines and the real-world data from the Vaccine Adverse Event Reporting System. Globally, our research found that the efficacy of all vaccines exceeded 70%, and RNA-based vaccines had the highest efficacy of 94.29%; moreover, Black or African American people, young people, and males may experience greater vaccine efficacy. The spectrum of vaccine-related adverse drug reactions (ADRs) is extremely broad, and the most frequent ADRs are pain, fatigue, and headache. Most ADRs are tolerable and are mainly grade 1 or 2 in severity. Some severe ADRs have been identified (thromboembolic events, 21-75 cases per million doses; myocarditis/pericarditis, 2-3 cases per million doses). In summary, vaccines are a powerful tool that can be used to control the COVID-19 pandemic, with high efficacy and tolerable ADRs. In addition, the spectrum of ADRs associated with the vaccines is broad, and most of the reactions appear within a week, although some may be delayed. Therefore, ADRs after vaccination need to be identified and addressed in a timely manner.
Keywords: COVID-19; SARS-CoV-2; efficacy; safety; vaccine.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



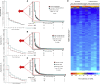
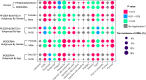
Comment in
-
The efficacy of COVID-19 vaccines against the B.1.617.2 (delta) variant.Mol Ther. 2021 Oct 6;29(10):2890-2892. doi: 10.1016/j.ymthe.2021.09.024. Epub 2021 Oct 2. Mol Ther. 2021. PMID: 34599870 Free PMC article. No abstract available.
References
-
- Organization W.H. 2021. WHO Coronavirus (COVID-19) Dashboard. World Health Organization.https://covid19.who.int/
-
- Organization W.H. 2021. Draft landscape and tracker of COVID-19 candidate vaccines.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
-
- Wikipedia . 2021. List of COVID-19 vaccine authorizations.https://en.wikipedia.org/w/index.php?title=List_of_COVID-19_vaccine_auth...
-
- Ritchie H., Ortiz-Ospina E., Beltekian D., Mathieu E., Hasell J., Macdonald B. 2021. Coronavirus (COVID-19) Vaccinations.https://ourworldindata.org/global-rise-of-education
-
- Ontario P.H. 2021. COVID-19 – What we know so far about herd immunity. Toronto, ON: Queen’s Printer for Ontario.https://www.publichealthontario.ca/en/diseases-and-conditions/infectious...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

