Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review
- PMID: 34365148
- PMCID: PMC8330139
- DOI: 10.1016/j.jns.2021.117607
Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review
Abstract
Introduction: The common reported adverse effects of COVID-19 vaccination consist of the injection site's local reaction followed by several non-specific flu-like symptoms. However, rare cases of vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) after viral vector vaccines (ChAdOx1 nCoV-19 vaccine, Ad26.COV2 vaccine) have been reported. Herein we systemically reviewed the reported cases of CVST and VITT following the COVID-19 vaccination.
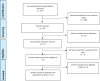
Methods: This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched PubMed until May 19, 2021, and the following Keywords were used: COVID Vaccine & Neurology, AstraZeneca COVID vaccine, ChAdOx1 nCoV-19 COVID vaccine, AZD1222 COVID vaccine, Janssen COVID vaccine, Johnson & Johnson COVID vaccine, Ad26.COV2 COVID vaccine. The authors evaluated the abstracts and titles of each article for screening and inclusion. English reports about post-vaccine CVST and VITT in humans were collected.
Results: Until May 19, we found 877 articles with the searched terms. We found 12 articles, which overall present clinical features of 36 patients with CVST and VITT after the ChAdOx1 nCoV-19 vaccine. Moreover, two articles were noted, which present 13 patients with CVST and VITT after Ad26.COV2 vaccine. The majority of the patients were females. Symptom onset occurred within one week after the first dose of vaccination (Range 4-19 days). Headache was the most common presenting symptom. Intracerebral hemorrhage (ICH) and/or Subarachnoid hemorrhage (SAH) were reported in 49% of the patients. The platelet count of the patients was between 5 and 127 cells×109/l, PF4 IgG Assay and d-Dimer were positive in the majority of the reported cases. Among 49 patients with CVST, at least 19 patients died (39%) due to complications of CVST and VITT.
Conclusion: Health care providers should be familiar with the clinical presentations, pathophysiology, diagnostic criteria, and management consideration of this rare but severe and potentially fatal complication of the COVID-19 vaccination. Early diagnosis and quick initiation of the treatment may help to provide patients with a more favorable neurological outcome.
Keywords: Ad26.COV2 vaccine; Cerebral venous sinus thrombosis; ChAdOx1 nCoV-19 vaccine; Vaccine-induced immune thrombotic thrombocytopenia.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

