Will the clinical development of 4th-generation "double mutant active" ALK TKIs (TPX-0131 and NVL-655) change the future treatment paradigm of ALK+ NSCLC?
- PMID: 34365220
- PMCID: PMC8353359
- DOI: 10.1016/j.tranon.2021.101191
Will the clinical development of 4th-generation "double mutant active" ALK TKIs (TPX-0131 and NVL-655) change the future treatment paradigm of ALK+ NSCLC?
Abstract
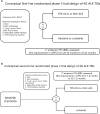
Our current treatment paradigm of advanced anaplastic lymphoma kinase fusion (ALK+) non-small cell lung cancer (NSCLC) classifies the six currently approved ALK tyrosine kinase inhibitors (TKIs) into three generations. The 2nd-generation (2G) and 3rd-generation (3G) ALK TKIs are all "single mutant active" with varying potencies across a wide spectrum of acquired single ALK resistance mutations. There is a vigorous debate among clinicians which is the best upfront ALK TKI is for the first-line (1L) treatment of ALK+ NSCLC and the subsequent sequencing strategies whether it should be based on the presence of specific on-target ALK resistance mutations or not. Regardless, sequential use of "single mutant active" ALK TKIs will eventually lead to double ALK resistance mutations in cis. This has led to the creation of fourth generation (4G) "double mutant active" ALK TKIs such as TPX-0131 and NVL-655. We discuss the critical properties 4G ALK TKIs must possess to be clinically successful. We proposed conceptual first-line, second-line, and molecularly-based third-line registrational randomized clinical trials designed for these 4G ALK TKIs. How these 4G ALK TKIs would be used in the future will depend on which line of treatment the clinical trial design(s) is adopted provided the trial is positive. If approved, 4G ALK TKIs may usher in a new treatment paradigm for advanced ALK+ NSCLC that is based on classifying ALK TKIs based on the intrinsic functional capabilities ("singe mutant active" versus "double mutant active") rather than the loosely-defined "generational" (first-, second-,third-,fourth-) classification and avoid the current clinical approaches of seemingly random sequential use of 2G and 3G ALK TKIs.
Keywords: 4th-generation ALK TKI; ALK+ NSCLC; Anaplastic lymphoma kinase tyrosine kinase inhibitors; NVL-655; TPX-0131; “Double mutant active”; “Single mutant active”.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Figures




References
-
- Nagasaka M., Ou S.I. Lorlatinib should be considered as the preferred first-line option in patients with advanced ALK-rearranged NSCLC. J. Thorac. Oncol. 2021 Apr;16(4):532–536. - PubMed
-
- Solomon B.J., Mok T., Kim D.W. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014 Dec 4;371(23):2167–2177. - PubMed
-
- Solomon B.J., Kim D.W., Wu Y.L. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J. Clin. Oncol. 2018 Aug 1;36(22):2251–2258. - PubMed
-
- Wu Y.L., Lu S., Lu Y. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J. Thorac. Oncol. 2018 Oct;13(10):1539–1548. - PubMed
-
- Soria J.C., Tan D.S.W., Chiari R. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomized, open-label, phase 3 study. Lancet. 2017 Mar 4;389(10072):917–929. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

