Nonhuman IAPP Variants Inhibit Human IAPP Aggregation
- PMID: 34365921
- PMCID: PMC10712300
- DOI: 10.2174/0929866528666210806152706
Nonhuman IAPP Variants Inhibit Human IAPP Aggregation
Abstract
Aim: To identify naturally occurring variants of IAPP capable of inhibiting the aggregation of human IAPP and protecting living cells from the toxic effects of human IAPP.
Background: The loss of insulin-producing β-cells and the overall progression of type 2 diabetes appears to be linked to the formation of toxic human IAPP (hIAPP, Islet Amyloid Polypeptide, amylin) amyloid in the pancreas. Inhibiting the initial aggregation of hIAPP has the potential to slow, if not stop entirely, the loss of β-cells and halt the progression of the disease.
Objective: To identify and characterize naturally occurring variants of IAPP capable of inhibiting human IAPP aggregation.
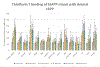
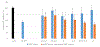
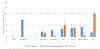
Methods: Synthetic human IAPP was incubated with synthetic IAPP variants identified from natural sources under conditions known to promote amyloid-based aggregation. To identify IAPP variants capable of inhibiting human IAPP aggregation, Thioflavin T-binding fluorescence, atomic force microscopy, and cell-rescue assays were performed.
Results: While most IAPP variants showed little to no ability to inhibit human IAPP aggregation, several variants showed some ability to inhibit aggregation, with two variants showing substantial inhibitory potential.
Conclusion: Several naturally occurring IAPP variants capable of inhibiting human IAPP aggregation were identified and characterized.
Keywords: Islet amyloid polypeptide; amylin; amyloid; pancreas.; protein aggregation; type 2 diabetes.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

