A Super-Resolved View of the Alzheimer's Disease-Related Amyloidogenic Pathway in Hippocampal Neurons
- PMID: 34366358
- PMCID: PMC8543249
- DOI: 10.3233/JAD-215008
A Super-Resolved View of the Alzheimer's Disease-Related Amyloidogenic Pathway in Hippocampal Neurons
Abstract
Background: Processing of the amyloid-β protein precursor (AβPP) is neurophysiologically important due to the resulting fragments that regulate synapse biology, as well as potentially harmful due to generation of the 42 amino acid long amyloid β-peptide (Aβ42), which is a key player in Alzheimer's disease.
Objective: Our aim was to clarify the subcellular locations of the fragments involved in the amyloidogenic pathway in primary neurons with a focus on Aβ42 and its immediate substrate AβPP C-terminal fragment (APP-CTF). To overcome the difficulties of resolving these compartments due to their small size, we used super-resolution microscopy.
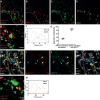
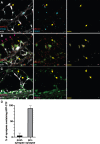
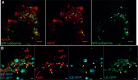
Methods: Mouse primary hippocampal neurons were immunolabelled and imaged by stimulated emission depletion (STED) microscopy, including three-dimensional three-channel imaging, and quantitative image analyses.
Results: The first (β-secretase) and second (γ-secretase) cleavages of AβPP were localized to functionally and distally distinct compartments. The β-secretase cleavage was observed in early endosomes in soma, where we were able to show that the liberated N- and C-terminal fragments were sorted into distinct vesicles budding from the early endosomes. Lack of colocalization of Aβ42 and APP-CTF in soma suggested that γ-secretase cleavage occurs in neurites. Indeed, APP-CTF was, in line with Aβ42 in our previous study, enriched in the presynapse but absent from the postsynapse. In contrast, full-length AβPP was not detected in either the pre- or the postsynaptic side of the synapse. Furthermore, we observed that endogenously produced and endocytosed Aβ42 were localized in different compartments.
Conclusion: These findings provide critical super-resolved insight into amyloidogenic AβPP processing in primary neurons.
Keywords: Alzheimer’s disease; Aβ42; amyloid-β protein precursor; stimulated emission depletion microscopy; synapse.
Conflict of interest statement
Authors’ disclosures available online (
Figures




References
-
- Söderberg L, Bogdanovic N, Axelsson B, Winblad B, Naslund J, Tjernberg LO (2006) Analysis of single Alzheimer solid plaque cores by laser capture microscopy and nanoelectrospray/tandem mass spectrometry. Biochemistry 45, 9849–9856. - PubMed
-
- Muller UC, Deller T, Korte M (2017) Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat Rev Neurosci 18, 281–298. - PubMed
-
- Jan A, Hartley DM, Lashuel HA (2010) Preparation and characterization of toxic Abeta aggregates for structural and functional studies in Alzheimer’s disease research. Nat Protoc 5, 1186–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

