Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion
- PMID: 34366380
- PMCID: PMC8609688
- DOI: 10.3233/JPD-212813
Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion
Abstract
Background: Foslevodopa/foscarbidopa, formerly known as ABBV-951, is a formulation of levodopa/carbidopa prodrugs with solubility that allows for subcutaneous (SC) infusion and is in development for the treatment of motor complications for patients with advanced Parkinson's disease (aPD).
Objective: The current work characterizes the levodopa (LD) and carbidopa (CD) pharmacokinetics (PK) following SC infusions of foslevodopa/foscarbidopa delivered at four different infusion rates in PD patients.
Methods: This was a Phase 1, single ascending dose, single-blind study conducted in 28 adult male and female subjects at seven sites in the United States. Foslevodopa/foscarbidopa was administered via abdominal SC infusion in PD patients over 72 hours. Patients were stratified in 4 groups and received a fixed dose of foslevodopa/foscarbidopa based on their oral daily LD intake. Serial plasma PK samples were collected to assay for LD and CD concentrations. Safety and tolerability were assessed throughout the study.
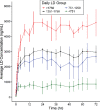
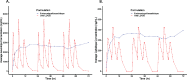
Results: LD exposure quickly reached steady state and remained stable with minimal fluctuations. Foslevodopa/foscarbidopa infusion provides stable LD and CD exposures compared to oral LD/CD dosing with the average steady-state exposure ranging from 747-4660 ng/mL for the different groups.
Conclusion: Foslevodopa/foscarbidopa was able to provide stable LD and CD exposures in PD patients over 72 hours via SC route of delivery with very low fluctuation in LD concentration level across a wide range of clinically relevant exposures. Foslevodopa/foscarbidopa had a favorable safety profile. The low PK fluctuation following foslevodopa/foscarbidopa infusion is expected to maintain LD exposure to treat aPD patients within a narrow therapeutic window.
Keywords: Levodopa; Parkinson’s disease; carbidopa; pharmacokinetics.
Conflict of interest statement
MR, WL, MN, and MFF are AbbVie employees and may own stock.
Figures


References
-
- Olanow CW, Watts RL, Koller WC (2001) An algorithm (decision tree) for the management of Parkinson’s disease (2001): Treatment guidelines. Neurology 56, S1–S88. - PubMed
-
- Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson’s disease: Scientific rationale and clinical implications. Lancet Neurol 5, 677–687. - PubMed
-
- Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A, Group LHS (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13, 141–149. - PMC - PubMed
-
- Yeh KC, August TF, Bush DF, Lasseter KC, Musson DG, Schwartz S, Smith ME, Titus DC (1989) Pharmacokinetics and bioavailability of Sinemet CR: A summary of human studies. Neurology 39, 25–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

