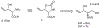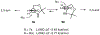Stereoselective Synthesis of (4 S,5 S)-5-Vinyloxazolidin-2-one-4-carboxylate as a β-Vinylserine Synthetic Equivalent by Vinyl Grignard Addition to an N-Tosyl Version of Garner's Aldehyde
- PMID: 34366570
- PMCID: PMC8341458
- DOI: 10.1055/a-1308-0370
Stereoselective Synthesis of (4 S,5 S)-5-Vinyloxazolidin-2-one-4-carboxylate as a β-Vinylserine Synthetic Equivalent by Vinyl Grignard Addition to an N-Tosyl Version of Garner's Aldehyde
Abstract
A highly efficient synthesis of a β-vinylserine synthetic equivalent is reported that exploits the stereodirecting effect of the N-toluenesulfonamide in an anti-diastereoselective (8.5:1) vinyl Grignard addition to an analogue of Garner's aldehyde. Both aryl and alkyl Grignards are shown to give increased anti-selectivity compared with N-Boc Garner's aldehyde.
Keywords: Garner aldehyde; alkenyl amino acid; oxazolidinones; vinylserine.
Figures
References
-
- Blackwell HE; Grubbs RH Angew. Chem. Int. Ed 1998, 37, 3281. - PubMed
- Schafmeister CE; Po J; Verdine GL J. Am. Chem. Soc 2000, 122, 5891.
- Aillard B; Robertson NS; Baldwin AR; Robins S; Jamieson AG Org. Biomol. Chem 2014, 12, 8775. - PubMed
- Yeo DJ; Warriner SL; Wilson AJ Chem. Commun 2013, 49, 9131. - PubMed
-
- Ibuka T; Habashita H; Otaka A; Fujii N; Oguchi Y; Uyehara T; Yamamoto YJ Org. Chem 1991, 56, 4370.
-
- Oishi S; Narumi T; Ohno H; Otaka A; Fujii N Yuki Gosei Kagaku Kyokaishi 2008, 66, 846.
- Venkatesan N; Kim BH Curr. Med. Chem 2002, 9, 2243. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources