A Combination of Cowpea Mosaic Virus and Immune Checkpoint Therapy Synergistically Improves Therapeutic Efficacy in Three Tumor Models
- PMID: 34366758
- PMCID: PMC8340625
- DOI: 10.1002/adfm.202002299
A Combination of Cowpea Mosaic Virus and Immune Checkpoint Therapy Synergistically Improves Therapeutic Efficacy in Three Tumor Models
Abstract
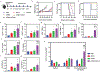
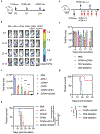
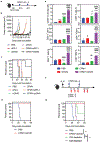
Immune checkpoint therapy (ICT) has the potential to treat cancer by removing the immunosuppressive brakes on T cell activity. However, ICT benefits only a subset of patients because most tumors are "cold", with limited pre-infiltration of effector T cells, poor immunogenicity, and low-level expression of checkpoint regulators. It has been previously reported that Cowpea mosaic virus (CPMV) promotes the activation of multiple innate immune cells and the secretion of pro-inflammatory cytokines to induce T cell cytotoxicity, suggesting that immunostimulatory CPMV could potentiate ICT. Here it is shown that in situ vaccination with CPMV increases the expression of checkpoint regulators on Foxp3-CD4+ effector T cells in the tumor microenvironment. It is shown that combined treatment with CPMV and selected checkpoint-targeting antibodies, specifically anti-PD-1 antibodies, or agonistic OX40-specific antibodies, reduced tumor burden, prolonged survival, and induced tumor antigen-specific immunologic memory to prevent relapse in three immunocompetent syngeneic mouse tumor models. This study therefore reveals new design principles for plant virus nanoparticles as novel immunotherapeutic adjuvants to elicit robust immune responses against cancer.
Keywords: Cowpea mosaic virus; immune checkpoint therapy; immunotherapy.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures

References
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Engl N. J. Med 2012, 366, 2443. - PMC - PubMed
-
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A, Nature 2014, 515, 568. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
