Probing the VIPR2 Microduplication Linkage to Schizophrenia in Animal and Cellular Models
- PMID: 34366784
- PMCID: PMC8339898
- DOI: 10.3389/fnins.2021.717490
Probing the VIPR2 Microduplication Linkage to Schizophrenia in Animal and Cellular Models
Abstract
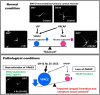
Pituitary adenylate cyclase-activating polypeptide (PACAP, gene name ADCYAP1) is a multifunctional neuropeptide involved in brain development and synaptic plasticity. With respect to PACAP function, most attention has been given to that mediated by its specific receptor PAC1 (ADCYAP1R1). However, PACAP also binds tightly to the high affinity receptors for vasoactive intestinal peptide (VIP, VIP), called VPAC1 and VPAC2 (VIPR1 and VIPR2, respectively). Depending on innervation patterns, PACAP can thus interact physiologically with any of these receptors. VPAC2 receptors, the focus of this review, are known to have a pivotal role in regulating circadian rhythms and to affect multiple other processes in the brain, including those involved in fear cognition. Accumulating evidence in human genetics indicates that microduplications at 7q36.3, containing VIPR2 gene, are linked to schizophrenia and possibly autism spectrum disorder. Although detailed molecular mechanisms have not been fully elucidated, recent studies in animal models suggest that overactivation of the VPAC2 receptor disrupts cortical circuit maturation. The VIPR2 linkage can thus be potentially explained by inappropriate control of receptor signaling at a time when neural circuits involved in cognition and social behavior are being established. Alternatively, or in addition, VPAC2 receptor overactivity may disrupt ongoing synaptic plasticity during processes of learning and memory. Finally, in vitro data indicate that PACAP and VIP have differential activities on the maturation of neurons via their distinct signaling pathways. Thus perturbations in the balance of VPAC2, VPAC1, and PAC1 receptors and their ligands may have important consequences in brain development and plasticity.
Keywords: VPAC2 receptor (VIPR2); cognition; neurodevelopment; psychiatric disorders; schizophrenia; synaptic plasticity.
Copyright © 2021 Ago, Asano, Hashimoto and Waschek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ago Y., Condro M. C., Tan Y. V., Ghiani C. A., Colwell C. S., Cushman J. D., et al. (2015). Reductions in synaptic proteins and selective alteration of prepulse inhibition in male C57BL/6 mice after postnatal administration of a VIP receptor (VIPR2) agonist. Psychopharmacology 232 2181–2189. 10.1007/s00213-014-3848-z - DOI - PMC - PubMed
-
- Ago Y., Hayata-Takano A., Kawanai T., Yamauchi R., Takeuchi S., Cushman J. D., et al. (2017). Impaired extinction of cued fear memory and abnormal dendritic morphology in the prelimbic and infralimbic cortices in VPAC2 receptor (VIPR2)-deficient mice. Neurobiol. Learn. Mem. 145 222–231. 10.1016/j.nlm.2017.10.010 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

