Clinically Used Hormone Formulations Differentially Impact Memory, Anxiety-Like, and Depressive-Like Behaviors in a Rat Model of Transitional Menopause
- PMID: 34366807
- PMCID: PMC8335488
- DOI: 10.3389/fnbeh.2021.696838
Clinically Used Hormone Formulations Differentially Impact Memory, Anxiety-Like, and Depressive-Like Behaviors in a Rat Model of Transitional Menopause
Abstract
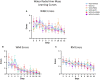
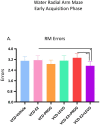
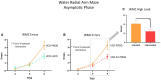
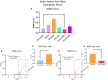
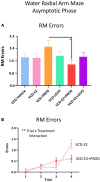
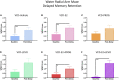
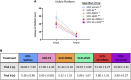
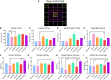
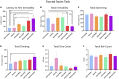
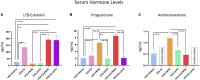
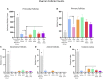
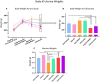
A variety of U.S. Food and Drug Administration-approved hormone therapy options are currently used to successfully alleviate unwanted symptoms associated with the changing endogenous hormonal milieu that occurs in midlife with menopause. Depending on the primary indication for treatment, different hormone therapy formulations are utilized, including estrogen-only, progestogen-only, or combined estrogen plus progestogen options. There is little known about how these formulations, or their unique pharmacodynamics, impact neurobiological processes. Seemingly disparate pre-clinical and clinical findings regarding the cognitive effects of hormone therapies, such as the negative effects associated with conjugated equine estrogens and medroxyprogesterone acetate vs. naturally circulating 17β-estradiol (E2) and progesterone, signal a critical need to further investigate the neuro-cognitive impact of hormone therapy formulations. Here, utilizing a rat model of transitional menopause, we administered either E2, progesterone, levonorgestrel, or combinations of E2 with progesterone or with levonorgestrel daily to follicle-depleted, middle-aged rats. A battery of assessments, including spatial memory, anxiety-like behaviors, and depressive-like behaviors, as well as endocrine status and ovarian follicle complement, were evaluated. Results indicate divergent outcomes for memory, anxiety, and depression, as well as unique physiological profiles, that were dependent upon the hormone regimen administered. Overall, the combination hormone treatments had the most consistently favorable profile for the domains evaluated in rats that had undergone experimentally induced transitional menopause and remained ovary-intact. The collective results underscore the importance of investigating variations in hormone therapy formulation as well as the menopause background upon which these formulations are delivered.
Keywords: VCD; anxiety; depression; estrogen; levonorgestrel; memory; menopause; progesterone.
Copyright © 2021 Koebele, Hiroi, Plumley, Melikian, Prakapenka, Patel, Carson, Kirby, Mennenga, Mayer, Dyer and Bimonte-Nelson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Acosta J. I., Mayer L., Talboom J. S., Tsang C. W. S., Smith C. J., Enders C. K., et al. (2009). Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology 150 4248–4259. 10.1210/en.2008-1802 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

