Switching and Discontinuation Pattern of Biologic Disease-Modifying Antirheumatic Drugs and Tofacitinib for Patients With Rheumatoid Arthritis in Taiwan
- PMID: 34366836
- PMCID: PMC8333863
- DOI: 10.3389/fphar.2021.628548
Switching and Discontinuation Pattern of Biologic Disease-Modifying Antirheumatic Drugs and Tofacitinib for Patients With Rheumatoid Arthritis in Taiwan
Abstract
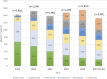
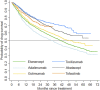
Rheumatoid arthritis (RA) is a chronic inflammatory systemic disease characterized by persistent joint synovial inflammation and swelling, leading to cartilage damage and bone erosion. This retrospective, longitudinal study is to evaluate the treatment patterns of biologic-naïve RA patients receiving index biologic disease-modifying antirheumatic drug (bDMARD) and tofacitinib by the data of Taiwan National Healthcare Insurance Claims and the Death Registry between 2012 and 2017. Drug survival and treatment patterns were determined by investigating the occurrence of switching and discontinuation from index treatment. At baseline, 70.0% of patients used tumor necrosis factor inhibitors (TNFi) bDMARD with the majority taking etanercept (27.0%) or adalimumab (26.2%). During the follow-up period, 40.0% (n = 3,464) of index users switched (n = 1,479) or discontinued (n = 1,985) the treatment with an average incidence rate of 0.18 per patient-year. Among the six index treatment groups, drug survival was the lowest for adalimumab and highest for tocilizumab. When compared with etanercept, only adalimumab had a higher cumulative probability of switching/discontinuation (adjusted HR = 1.17, 95% CI: 1.08-1.28), whereas golimumab, non-TNFi bDMARDs and tofacitinib were significantly less probable to switch or discontinue. For patients switching the index treatment, tocilizumab (31.2%) and tofacitinib (23.4%) were the main regimens being switched to. In addition, 48.2% of patients who discontinued the index treatment received further retreatment, and 63.8-77.0% of them were retreated with same agent. In conclusion, this population-based study found that TNFi were the preferred agents as the index treatments during 2012-2017. Non-TNFi and tofacitinib were more common second-line agents being switched to. Nearly half of discontinued patients received retreatment, with a majority receiving the same agent.
Keywords: biologic disease-modifying antirheumatic drug; rheumatoid arthritis; tofacitinib; treatment pattern; tumor necrosis factor inhibitors.
Copyright © 2021 Li, Chang, Hsin and Tang.
Conflict of interest statement
K-JL has received speaker fees from Pfizer, Abbvie, Roche, Lilly, Janssen, Chugai, and Novartis (less than $10,000 each). C-YH is an employee of Pfizer. No other disclosures relevant to this article were reported. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Incidence and risk factors of discontinuation of tofacitinib and biologic disease-modifying anti-rheumatic drugs among patients with rheumatoid arthritis: A population-based cohort study.Clin Rheumatol. 2024 Dec;43(12):3625-3637. doi: 10.1007/s10067-024-07161-6. Epub 2024 Oct 11. Clin Rheumatol. 2024. PMID: 39392514
-
Two-year persistence of golimumab as second-line biologic agent in rheumatoid arthritis as compared to other subcutaneous tumor necrosis factor inhibitors: real-life data from the LORHEN registry.Int J Rheum Dis. 2018 Feb;21(2):422-430. doi: 10.1111/1756-185X.13199. Epub 2017 Oct 30. Int J Rheum Dis. 2018. PMID: 29082659
-
Association of first, second, and third-line bDMARDs and tsDMARD with drug survival among seropositive rheumatoid arthritis patients: Cohort study in A real world setting.Semin Arthritis Rheum. 2021 Aug;51(4):685-691. doi: 10.1016/j.semarthrit.2021.06.002. Epub 2021 Jun 7. Semin Arthritis Rheum. 2021. PMID: 34139521
-
Economic Burden of Switching to a Non-Tumor Necrosis Factor Inhibitor Versus a Tumor Necrosis Factor Inhibitor Biologic Therapy among Patients with Rheumatoid Arthritis.Adv Ther. 2016 May;33(5):807-23. doi: 10.1007/s12325-016-0318-5. Epub 2016 Mar 24. Adv Ther. 2016. PMID: 27084724
-
Switching biologics in the treatment of psoriatic arthritis.Semin Arthritis Rheum. 2017 Aug;47(1):29-37. doi: 10.1016/j.semarthrit.2017.02.001. Epub 2017 Feb 8. Semin Arthritis Rheum. 2017. PMID: 28363434 Review.
Cited by
-
Treatment Sequence After Initiating Biologic Therapy for Patients With Rheumatoid Arthritis in Korea: A Nationwide Retrospective Cohort Study.J Rheum Dis. 2023 Jan 1;30(1):26-35. doi: 10.4078/jrd.22.0024. Epub 2022 Aug 30. J Rheum Dis. 2023. PMID: 37476522 Free PMC article.
-
Incidence and risk factors of discontinuation of tofacitinib and biologic disease-modifying anti-rheumatic drugs among patients with rheumatoid arthritis: A population-based cohort study.Clin Rheumatol. 2024 Dec;43(12):3625-3637. doi: 10.1007/s10067-024-07161-6. Epub 2024 Oct 11. Clin Rheumatol. 2024. PMID: 39392514
-
Real-world treatment patterns of rheumatoid arthritis in Brazil: analysis of DATASUS national administrative claims data for pharmacoepidemiology studies (2010-2020).Sci Rep. 2023 Oct 18;13(1):17739. doi: 10.1038/s41598-023-44389-9. Sci Rep. 2023. PMID: 37853013 Free PMC article.
-
Real-world effectiveness of biological therapy in patients with rheumatoid arthritis: Systematic review and meta-analysis.Front Pharmacol. 2022 Aug 11;13:927179. doi: 10.3389/fphar.2022.927179. eCollection 2022. Front Pharmacol. 2022. PMID: 36034836 Free PMC article.
-
Management and treatment outcomes of rheumatoid arthritis in the era of biologic and targeted synthetic therapies: evaluation of 10-year data from the KURAMA cohort.Arthritis Res Ther. 2024 Jan 9;26(1):16. doi: 10.1186/s13075-023-03251-z. Arthritis Res Ther. 2024. PMID: 38195572 Free PMC article.
References
-
- Bureau of National Health Insuarance (2013). Provision of the Coverage and Reimbursement Policies/Criteria for Immunologic Agents [Chinese]. Available from: https://www.nhi.gov.tw/Content_List.aspx?n=2A21BF7EBFA32789=5FE8C9FEAE86....
-
- Cannon G. W., DuVall S. L., Haroldsen C. L., Caplan L., Curtis J. R., Michaud K., et al. (2016). Clinical Outcomes and Biologic Costs of Switching between Tumor Necrosis Factor Inhibitors in US Veterans with Rheumatoid Arthritis. Adv. Ther. 33 (8), 1347–1359. 10.1007/s12325-016-0371-0 - DOI - PMC - PubMed
-
- Caporali R., Zavaglia D. (2019). Real-world Experience with Tofacitinib for the Treatment of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 37 (3), 485–495. 30183607 - PubMed
LinkOut - more resources
Full Text Sources

