Switching and Discontinuation Pattern of Biologic Disease-Modifying Antirheumatic Drugs and Tofacitinib for Patients With Rheumatoid Arthritis in Taiwan
- PMID: 34366836
- PMCID: PMC8333863
- DOI: 10.3389/fphar.2021.628548
Switching and Discontinuation Pattern of Biologic Disease-Modifying Antirheumatic Drugs and Tofacitinib for Patients With Rheumatoid Arthritis in Taiwan
Abstract
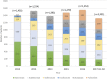
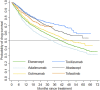
Rheumatoid arthritis (RA) is a chronic inflammatory systemic disease characterized by persistent joint synovial inflammation and swelling, leading to cartilage damage and bone erosion. This retrospective, longitudinal study is to evaluate the treatment patterns of biologic-naïve RA patients receiving index biologic disease-modifying antirheumatic drug (bDMARD) and tofacitinib by the data of Taiwan National Healthcare Insurance Claims and the Death Registry between 2012 and 2017. Drug survival and treatment patterns were determined by investigating the occurrence of switching and discontinuation from index treatment. At baseline, 70.0% of patients used tumor necrosis factor inhibitors (TNFi) bDMARD with the majority taking etanercept (27.0%) or adalimumab (26.2%). During the follow-up period, 40.0% (n = 3,464) of index users switched (n = 1,479) or discontinued (n = 1,985) the treatment with an average incidence rate of 0.18 per patient-year. Among the six index treatment groups, drug survival was the lowest for adalimumab and highest for tocilizumab. When compared with etanercept, only adalimumab had a higher cumulative probability of switching/discontinuation (adjusted HR = 1.17, 95% CI: 1.08-1.28), whereas golimumab, non-TNFi bDMARDs and tofacitinib were significantly less probable to switch or discontinue. For patients switching the index treatment, tocilizumab (31.2%) and tofacitinib (23.4%) were the main regimens being switched to. In addition, 48.2% of patients who discontinued the index treatment received further retreatment, and 63.8-77.0% of them were retreated with same agent. In conclusion, this population-based study found that TNFi were the preferred agents as the index treatments during 2012-2017. Non-TNFi and tofacitinib were more common second-line agents being switched to. Nearly half of discontinued patients received retreatment, with a majority receiving the same agent.
Keywords: biologic disease-modifying antirheumatic drug; rheumatoid arthritis; tofacitinib; treatment pattern; tumor necrosis factor inhibitors.
Copyright © 2021 Li, Chang, Hsin and Tang.
Conflict of interest statement
K-JL has received speaker fees from Pfizer, Abbvie, Roche, Lilly, Janssen, Chugai, and Novartis (less than $10,000 each). C-YH is an employee of Pfizer. No other disclosures relevant to this article were reported. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bureau of National Health Insuarance (2013). Provision of the Coverage and Reimbursement Policies/Criteria for Immunologic Agents [Chinese]. Available from: https://www.nhi.gov.tw/Content_List.aspx?n=2A21BF7EBFA32789=5FE8C9FEAE86....
-
- Cannon G. W., DuVall S. L., Haroldsen C. L., Caplan L., Curtis J. R., Michaud K., et al. (2016). Clinical Outcomes and Biologic Costs of Switching between Tumor Necrosis Factor Inhibitors in US Veterans with Rheumatoid Arthritis. Adv. Ther. 33 (8), 1347–1359. 10.1007/s12325-016-0371-0 - DOI - PMC - PubMed
-
- Caporali R., Zavaglia D. (2019). Real-world Experience with Tofacitinib for the Treatment of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 37 (3), 485–495. 30183607 - PubMed
LinkOut - more resources
Full Text Sources

