Effect of Dysferlin Deficiency on Atherosclerosis and Plasma Lipoprotein Composition Under Normal and Hyperlipidemic Conditions
- PMID: 34366880
- PMCID: PMC8339577
- DOI: 10.3389/fphys.2021.675322
Effect of Dysferlin Deficiency on Atherosclerosis and Plasma Lipoprotein Composition Under Normal and Hyperlipidemic Conditions
Abstract
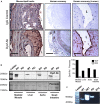
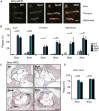
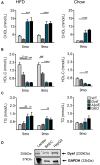
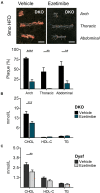
Dysferlinopathies are a group of muscle disorders caused by mutations to dysferlin, a transmembrane protein involved in membrane patching events following physical damage to skeletal myofibers. We documented dysferlin expression in vascular tissues including non-muscle endothelial cells, suggesting that blood vessels may have an endogenous repair system that helps promote vascular homeostasis. To test this hypothesis, we generated dysferlin-null mice lacking apolipoprotein E (ApoE), a common model of atherosclerosis, dyslipidemia and endothelial injury when stressed with a high fat, and cholesterol-rich diet. Despite high dysferlin expression in mouse and human atheromatous plaques, loss of dysferlin did not affect atherosclerotic burden as measured in the aortic root, arch, thoracic, and abdominal aortic regions. Interestingly, we observed that dysferlin-null mice exhibit lower plasma high-density lipoprotein cholesterol (HDL-C) levels than their WT controls at all measured stages of the disease process. Western blotting revealed abundant dysferlin expression in protein extracts from mouse livers, the main regulator of plasma lipoprotein levels. Despite abnormal lipoprotein levels, Dysf/ApoE double knockout mice responded to cholesterol absorption blockade with lower total cholesterol and blunted atherosclerosis. Our study suggests that dysferlin does not protect against atherosclerosis or participate in cholesterol absorption blockade but regulates basal plasma lipoprotein composition. Dysferlinopathic patients may be dyslipidemic without greater atherosclerotic burden while remaining responsive to cholesterol absorption blockade.
Keywords: atherosclerosis; dysferlin; endothelial function; hyperlipidemia; lipids; plaque; vascular homeostasis.
Copyright © 2021 White, Milad, Sellers and Bernatchez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Increased nonHDL cholesterol levels cause muscle wasting and ambulatory dysfunction in the mouse model of LGMD2B.J Lipid Res. 2018 Feb;59(2):261-272. doi: 10.1194/jlr.M079459. Epub 2017 Nov 25. J Lipid Res. 2018. PMID: 29175948 Free PMC article.
-
Increased plasma lipid levels exacerbate muscle pathology in the mdx mouse model of Duchenne muscular dystrophy.Skelet Muscle. 2017 Sep 12;7(1):19. doi: 10.1186/s13395-017-0135-9. Skelet Muscle. 2017. PMID: 28899419 Free PMC article.
-
DYSF promotes monocyte activation in atherosclerotic cardiovascular disease as a DNA methylation-driven gene.Transl Res. 2022 Sep;247:19-38. doi: 10.1016/j.trsl.2022.04.001. Epub 2022 Apr 20. Transl Res. 2022. PMID: 35460889
-
Standardized fraction of Xylocarpus moluccensis fruits improve vascular relaxation and plaque stability in dyslipidemic models of atherosclerosis.J Ethnopharmacol. 2018 Mar 1;213:81-91. doi: 10.1016/j.jep.2017.11.004. Epub 2017 Nov 10. J Ethnopharmacol. 2018. PMID: 29129602
-
A promotive effect for halofuginone on membrane repair and synaptotagmin-7 levels in muscle cells of dysferlin-null mice.Hum Mol Genet. 2018 Aug 15;27(16):2817-2829. doi: 10.1093/hmg/ddy185. Hum Mol Genet. 2018. PMID: 29771357
Cited by
-
Mechanisms of Endothelial Cell Membrane Repair: Progress and Perspectives.Cells. 2023 Nov 17;12(22):2648. doi: 10.3390/cells12222648. Cells. 2023. PMID: 37998383 Free PMC article. Review.
-
High-Density Lipoprotein-Associated Cholesterol Abnormalities in a Clinical Outcomes Study of Dysferlin-Deficient Limb-Girdle Muscular Dystrophy Type R2.J Cachexia Sarcopenia Muscle. 2025 Aug;16(4):e70042. doi: 10.1002/jcsm.70042. J Cachexia Sarcopenia Muscle. 2025. PMID: 40814795 Free PMC article.
-
Identification of macrophage-related genes in sepsis-induced ARDS using bioinformatics and machine learning.Sci Rep. 2023 Jun 19;13(1):9876. doi: 10.1038/s41598-023-37162-5. Sci Rep. 2023. PMID: 37336980 Free PMC article.
-
Fibro-adipogenic progenitors in physiological adipogenesis and intermuscular adipose tissue remodeling.Mol Aspects Med. 2024 Jun;97:101277. doi: 10.1016/j.mam.2024.101277. Epub 2024 May 23. Mol Aspects Med. 2024. PMID: 38788527 Free PMC article. Review.
-
Limb-girdle muscular dystrophy type 2B causes HDL-C abnormalities in patients and statin-resistant muscle wasting in dysferlin-deficient mice.Skelet Muscle. 2022 Nov 29;12(1):25. doi: 10.1186/s13395-022-00308-6. Skelet Muscle. 2022. PMID: 36447272 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

