New Frontiers for Deep Brain Stimulation: Directionality, Sensing Technologies, Remote Programming, Robotic Stereotactic Assistance, Asleep Procedures, and Connectomics
- PMID: 34367055
- PMCID: PMC8340024
- DOI: 10.3389/fneur.2021.694747
New Frontiers for Deep Brain Stimulation: Directionality, Sensing Technologies, Remote Programming, Robotic Stereotactic Assistance, Asleep Procedures, and Connectomics
Abstract
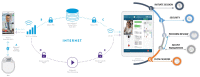
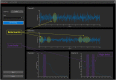
Over the last few years, while expanding its clinical indications from movement disorders to epilepsy and psychiatry, the field of deep brain stimulation (DBS) has seen significant innovations. Hardware developments have introduced directional leads to stimulate specific brain targets and sensing electrodes to determine optimal settings via feedback from local field potentials. In addition, variable-frequency stimulation and asynchronous high-frequency pulse trains have introduced new programming paradigms to efficiently desynchronize pathological neural circuitry and regulate dysfunctional brain networks not responsive to conventional settings. Overall, these innovations have provided clinicians with more anatomically accurate programming and closed-looped feedback to identify optimal strategies for neuromodulation. Simultaneously, software developments have simplified programming algorithms, introduced platforms for DBS remote management via telemedicine, and tools for estimating the volume of tissue activated within and outside the DBS targets. Finally, the surgical accuracy has improved thanks to intraoperative magnetic resonance or computerized tomography guidance, network-based imaging for DBS planning and targeting, and robotic-assisted surgery for ultra-accurate, millimetric lead placement. These technological and imaging advances have collectively optimized DBS outcomes and allowed "asleep" DBS procedures. Still, the short- and long-term outcomes of different implantable devices, surgical techniques, and asleep vs. awake procedures remain to be clarified. This expert review summarizes and critically discusses these recent innovations and their potential impact on the DBS field.
Keywords: asleep; connectomics; deep brain stimulation; directionality; local field potential; robotic surgery; sensing; telemedicine.
Copyright © 2021 Merola, Singh, Reeves, Changizi, Goetz, Rossi, Pallavaram, Carcieri, Harel, Shaikhouni, Sammartino, Krishna, Verhagen and Dalm.
Conflict of interest statement
AM has received support from the NIH (KL2 TR001426), speaker honoraria from CSL Behring, Abbvie, Abbott, Theravance, and Cynapsus Therapeutics. He has received a salary as chief Editor of Frontiers in Neurology, Experimental Therapeutics, and grant support from Lundbeck and Abbvie. BC has received speaker honoraria from Abbvie. SG is an employee at Medtronic. LR is an employee and shareholder in Newronika, Inc. SP is an employee of Abbott Laboratories. SC is an employee of Boston Scientific. NH is co-founder, and shareholder in Surgical Information Sciences, Inc. VK has received grant support from Medtronic. LV is an editorial board member of Neurology and Therapy, and Brain Sciences. He has received consultant honoraria from Abbott, AbbVie Inc, and Boston Scientific, and research support from Medtronic, Boston Scientific, Abbott, AbbVie, Neuroderm, Biogen Inc. He has received NIH funding (R01 NS40902) as a site-PI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Deep brain stimulation: new programming algorithms and teleprogramming.Expert Rev Neurother. 2023 May;23(5):467-478. doi: 10.1080/14737175.2023.2208749. Epub 2023 May 3. Expert Rev Neurother. 2023. PMID: 37115193 Review.
-
Electrode Placement Accuracy in Robot-Assisted Asleep Deep Brain Stimulation.Ann Biomed Eng. 2019 May;47(5):1212-1222. doi: 10.1007/s10439-019-02230-3. Epub 2019 Feb 22. Ann Biomed Eng. 2019. PMID: 30796551 Clinical Trial.
-
Deep brain stimulation programming strategies: segmented leads, independent current sources, and future technology.Expert Rev Med Devices. 2021 Sep;18(9):875-891. doi: 10.1080/17434440.2021.1962286. Epub 2021 Sep 6. Expert Rev Med Devices. 2021. PMID: 34329566 Review.
-
Technological Advances in Deep Brain Stimulation.J Parkinsons Dis. 2015;5(3):483-96. doi: 10.3233/JPD-150579. J Parkinsons Dis. 2015. PMID: 26406128 Review.
-
Technological advances in the surgical treatment of movement disorders.Curr Neurol Neurosci Rep. 2013 Aug;13(8):371. doi: 10.1007/s11910-013-0371-2. Curr Neurol Neurosci Rep. 2013. PMID: 23812894 Free PMC article. Review.
Cited by
-
An update on advanced therapies for Parkinson's disease: From gene therapy to neuromodulation.Front Surg. 2022 Sep 23;9:863921. doi: 10.3389/fsurg.2022.863921. eCollection 2022. Front Surg. 2022. PMID: 36211256 Free PMC article. Review.
-
Future directions in psychiatric neurosurgery: Proceedings of the 2022 American Society for Stereotactic and Functional Neurosurgery meeting on surgical neuromodulation for psychiatric disorders.Brain Stimul. 2023 May-Jun;16(3):867-878. doi: 10.1016/j.brs.2023.05.011. Epub 2023 May 20. Brain Stimul. 2023. PMID: 37217075 Free PMC article.
-
Patient, target, device, and program selection for DBS in Parkinson's disease: advancing toward precision care.NPJ Parkinsons Dis. 2025 Jul 1;11(1):195. doi: 10.1038/s41531-025-01015-x. NPJ Parkinsons Dis. 2025. PMID: 40595684 Free PMC article. Review.
-
Deep brain stimulation in Parkinson's disease: state of the art and future perspectives.Arq Neuropsiquiatr. 2022 May;80(5 Suppl 1):105-115. doi: 10.1590/0004-282X-ANP-2022-S133. Arq Neuropsiquiatr. 2022. PMID: 35976323 Free PMC article. Review.
-
Research status and hotspots in the surgical treatment of tremor in Parkinson's disease from 2002 to 2022: a bibliometric and visualization analysis.Front Aging Neurosci. 2023 Sep 27;15:1157443. doi: 10.3389/fnagi.2023.1157443. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37829141 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

