The Molecular Mechanism of Sex Hormones on Sertoli Cell Development and Proliferation
- PMID: 34367061
- PMCID: PMC8344352
- DOI: 10.3389/fendo.2021.648141
The Molecular Mechanism of Sex Hormones on Sertoli Cell Development and Proliferation
Abstract
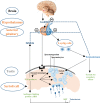
Sustaining and maintaining the intricate process of spermatogenesis is liable upon hormones and growth factors acting through endocrine and paracrine pathways. The Sertoli cells (SCs) are the major somatic cells present in the seminiferous tubules and are considered to be the main regulators of spermatogenesis. As each Sertoli cell supports a specific number of germ cells, thus, the final number of Sertoli cells determines the sperm production capacity. Similarly, sex hormones are also major regulators of spermatogenesis and they can determine the proliferation of Sertoli cells. In the present review, we have critically and comprehensively discussed the role of sex hormones and some other factors that are involved in Sertoli cell proliferation, differentiation and maturation. Furthermore, we have also presented a model of Sertoli cell development based upon the recent advancement in the field of reproduction. Hence, our review article provides a general overview regarding the sex hormonal pathways governing Sertoli cell proliferation and development.
Keywords: Sertoli cells; fertility; sex hormone; spermatogenesis; testis.
Copyright © 2021 Shah, Khan, Shah, Khan, Dil, Liu, Wen and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice.Mol Endocrinol. 2013 May;27(5):814-27. doi: 10.1210/me.2012-1258. Epub 2013 Mar 21. Mol Endocrinol. 2013. PMID: 23518924 Free PMC article.
-
Molecular insights into hormone regulation via signaling pathways in Sertoli cells: With discussion on infertility and testicular tumor.Gene. 2020 Aug 30;753:144812. doi: 10.1016/j.gene.2020.144812. Epub 2020 May 26. Gene. 2020. PMID: 32470507 Review.
-
The Sertoli cell as the orchestra conductor of spermatogenesis: spermatogenic cells dance to the tune of testosterone.Hormones (Athens). 2015 Oct-Dec;14(4):479-503. doi: 10.14310/horm.2002.1633. Hormones (Athens). 2015. PMID: 26732153 Review.
-
Hormonal control of germ cell development and spermatogenesis.Semin Cell Dev Biol. 2014 May;29:55-65. doi: 10.1016/j.semcdb.2014.02.010. Epub 2014 Mar 2. Semin Cell Dev Biol. 2014. PMID: 24598767 Review.
-
Mechanisms of hormonal regulation of sertoli cell development and proliferation: a key process for spermatogenesis.Curr Mol Pharmacol. 2014;7(2):96-108. doi: 10.2174/1874467208666150126155032. Curr Mol Pharmacol. 2014. PMID: 25620228 Review.
Cited by
-
Oxidative Stress-induced Hormonal Disruption in Male Reproduction.Reprod Sci. 2024 Oct;31(10):2943-2956. doi: 10.1007/s43032-024-01662-0. Epub 2024 Aug 1. Reprod Sci. 2024. PMID: 39090335 Review.
-
Effects of the low-carb organic Mediterranean diet on testosterone levels and sperm DNA fragmentation.Curr Res Food Sci. 2023 Nov 15;7:100636. doi: 10.1016/j.crfs.2023.100636. eCollection 2023. Curr Res Food Sci. 2023. PMID: 38045510 Free PMC article.
-
MAEL gene contributes to bovine testicular development through the m5C-mediated splicing.iScience. 2023 Jan 6;26(2):105941. doi: 10.1016/j.isci.2023.105941. eCollection 2023 Feb 17. iScience. 2023. PMID: 36711243 Free PMC article.
-
Evaluation of semen parameters and histopathological comparison of inguinal and abdominal orchiectomy specimens in late post pubertal men with unilateral undescended testis.Basic Clin Androl. 2025 Jun 17;35(1):23. doi: 10.1186/s12610-025-00270-5. Basic Clin Androl. 2025. PMID: 40528178 Free PMC article.
-
The construction of a testis transcriptional cell atlas from embryo to adult reveals various somatic cells and their molecular roles.J Transl Med. 2023 Nov 27;21(1):859. doi: 10.1186/s12967-023-04722-2. J Transl Med. 2023. PMID: 38012716 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

