Glycosphingolipids in Filamentous Fungi: Biological Roles and Potential Applications in Cosmetics and Health Foods
- PMID: 34367090
- PMCID: PMC8341767
- DOI: 10.3389/fmicb.2021.690211
Glycosphingolipids in Filamentous Fungi: Biological Roles and Potential Applications in Cosmetics and Health Foods
Abstract
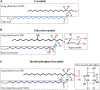
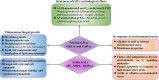
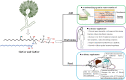
Filamentous fungi are a group of economically important fungi used in the production of fermented foods, industrial enzymes, and secondary metabolites. Glycosphingolipids (GSLs) as constituents of lipid rafts are involved in growth, differentiation, and response to environment stress in filamentous fungi. In addition to these key roles, GSLs are also important in the barrier function of skin to retain moisture as a moisturizing ingredient in cosmetics or health products for their strong biological activity as a functional component. GSLs found in filamentous fungi are divided in two major classes: neutral GSLs (glycosylceramides), glucosylceramides (GlcCers), and/or galactosylceramides (GalCers) and acidic GSLs, mannosylinositol phosphorylceramide (MIPC) and mannosyldiinositol phosphorylceramide [M(IP)2C]. Glycosylceramides are one of the abundant GSLs in Aspergillus and known to improve skin-barrier function and prevent intestinal impairment as a prebiotic. Some filamentous fungi of Aspergillus spp., synthesizing both GlcCer and GalCer, would be an amenable source to exploit glycosylceramides that wildly adding in cosmetics as moisturizing ingredients or health food as dietary supplements. In this minireview, the types, structures, and biosynthetic pathways of GSLs in filamentous fungi, and the relevance of GSLs in fungal growth, spore formation, and environmental stress response are explained. Furthermore, the advantage, potential development, and application of GlcCer and GalCer from filamentous fungi Aspergillus spp. are also investigate based on the use of plant GlcCer in health foods and cosmetics.
Keywords: Aspergillus; application of glycosylceramide; biosynthetic pathway; filamentous fungi; glucosylceramide and galactosylceramide; glycosphingolipids.
Copyright © 2021 Jiang, Ge, He and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Biological Roles Played by Sphingolipids in Dimorphic and Filamentous Fungi.mBio. 2018 May 15;9(3):e00642-18. doi: 10.1128/mBio.00642-18. mBio. 2018. PMID: 29764947 Free PMC article. Review.
-
Structural diversity and biological significance of glycosphingolipids in pathogenic and opportunistic fungi.Front Cell Infect Microbiol. 2014 Sep 25;4:138. doi: 10.3389/fcimb.2014.00138. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25309884 Free PMC article. Review.
-
Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus.Biochemistry. 1999 Jun 1;38(22):7294-306. doi: 10.1021/bi982898z. Biochemistry. 1999. PMID: 10353841
-
Glucosylceramide and galactosylceramide, small glycosphingolipids with significant impact on health and disease.Glycobiology. 2021 Dec 18;31(11):1416-1434. doi: 10.1093/glycob/cwab046. Glycobiology. 2021. PMID: 34080016 Free PMC article. Review.
-
Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa.Microorganisms. 2023 Aug 16;11(8):2093. doi: 10.3390/microorganisms11082093. Microorganisms. 2023. PMID: 37630653 Free PMC article.
Cited by
-
Access to Anti-Biofilm Compounds from Endolichenic Fungi Using a Bioguided Networking Screening.J Fungi (Basel). 2022 Sep 27;8(10):1012. doi: 10.3390/jof8101012. J Fungi (Basel). 2022. PMID: 36294577 Free PMC article.
-
The role and mechanisms of cordycepin in inhibiting cancer cells.Braz J Med Biol Res. 2024 Aug 23;57:e13889. doi: 10.1590/1414-431X2024e13889. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 39194034 Free PMC article. Review.
-
Two structurally different oomycete lipophilic microbe-associated molecular patterns induce distinctive plant immune responses.Plant Physiol. 2024 Sep 2;196(1):479-494. doi: 10.1093/plphys/kiae255. Plant Physiol. 2024. PMID: 38828881 Free PMC article.
-
Functional study of two ER localized sterol C-14 reductases in Aspergillus oryzae.3 Biotech. 2024 May;14(5):136. doi: 10.1007/s13205-024-03988-7. Epub 2024 Apr 25. 3 Biotech. 2024. PMID: 38682096 Free PMC article.
-
Pilocarpine inhibits Candida albicans SC5314 biofilm maturation by altering lipid, sphingolipid, and protein content.Microbiol Spectr. 2025 May 6;13(5):e0298724. doi: 10.1128/spectrum.02987-24. Epub 2025 Mar 20. Microbiol Spectr. 2025. PMID: 40111054 Free PMC article.
References
-
- Aida K., Kinoshita M., Sugawara T., Ono J., Miyazawa T., Ohnishi M. (2004). Apoptosis inducement by plant and fungus sphingoid bases in human colon cancer cells. J. Oleo Sci. 53 503–510. 10.5650/jos.53.503 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

