Effect of Osmotic Stress on the Growth, Development and Pathogenicity of Setosphaeria turcica
- PMID: 34367108
- PMCID: PMC8342955
- DOI: 10.3389/fmicb.2021.706349
Effect of Osmotic Stress on the Growth, Development and Pathogenicity of Setosphaeria turcica
Abstract
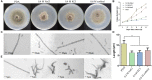
Osmotic stress is a severe condition frequently encountered by microorganisms; however, there is limited knowledge on the influence of hyperosmotic stress on the growth, development and pathogenicity of phytopathogenic fungi. Here, three osmotic conditions (0.4 M NaCl, 0.4 M KCl, and 0.6 M sorbitol supplemented in potato dextrose agar medium) were used to identify the effect of osmotic stress on the growth, development and pathogenicity of Setosphaeria turcica which is a plant pathogenic fungus and causes northern corn leaf blight disease in maize, sorghum, and related grasses. In osmotic stress, the growth rate of mycelium was decreased, and the number of vesicular structures and flocculent secretion outside the hypha cell wall were significantly increased. The qRT-PCR results showed that the osmotic stress quickly activated the HOG-MAPK pathway, up-regulated the expression of the downstream genes, and these genes were most highly expressed within 30 min of exposure to osmotic stress. Furthermore, the germination rate and the yield of conidia were significantly higher under osmotic stress than in the control. A pathogenicity analysis confirmed that pathogenicity of the conidia which were cultured under osmotic stress was significantly enhanced. By analyzing the knock-out mutants of an osmotic stress responsed gene StFPS1, an aquaglyceroporin downstream of the HOG-MAPK pathway, we found that StFPS1 was involved in the formation of appressorium and penetration peg, which affected the penetration ability of S. turcica. In summary, our work explained the correlation between osmotic stress and growth, development, and pathogenicity in S. turcica.
Keywords: HOG-MAPK; Setosphaeria turcica; StFPS1; osmotic; pathogenicity.
Copyright © 2021 Liu, Gong, Li, Si, Zhou, Liu, Fan, Zhang, Han, Gu and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
StRAB4 gene is required for filamentous growth, conidial development, and pathogenicity in Setosphaeria turcica.Front Microbiol. 2024 Jan 8;14:1302081. doi: 10.3389/fmicb.2023.1302081. eCollection 2023. Front Microbiol. 2024. PMID: 38264490 Free PMC article.
-
Novel factors contributing to fungal pathogenicity at early stages of Setosphaeria turcica infection.Mol Plant Pathol. 2022 Jan;23(1):32-44. doi: 10.1111/mpp.13140. Epub 2021 Oct 10. Mol Plant Pathol. 2022. PMID: 34628700 Free PMC article.
-
The Septin Gene StSep4 Contributes to the Pathogenicity of Setosphaeria turcica by Regulating the Morphology, Cell Wall Integrity, and Pathogenic Factor Biosynthesis.J Agric Food Chem. 2023 Dec 13;71(49):19568-19580. doi: 10.1021/acs.jafc.3c06635. Epub 2023 Nov 29. J Agric Food Chem. 2023. PMID: 38019936
-
Genetics of Resistance and Pathogenicity in the Maize/Setosphaeria turcica Pathosystem and Implications for Breeding.Front Plant Sci. 2017 Aug 29;8:1490. doi: 10.3389/fpls.2017.01490. eCollection 2017. Front Plant Sci. 2017. PMID: 28900437 Free PMC article. Review.
-
Debaryomyces hansenii, a highly osmo-tolerant and halo-tolerant yeast, maintains activated Dhog1p in the cytoplasm during its growth under severe osmotic stress.Curr Genet. 2005 Sep;48(3):162-70. doi: 10.1007/s00294-005-0010-9. Epub 2005 Oct 12. Curr Genet. 2005. PMID: 16091960 Review.
Cited by
-
Fungal Stress Responses and the Importance of GPCRs.J Fungi (Basel). 2025 Mar 11;11(3):213. doi: 10.3390/jof11030213. J Fungi (Basel). 2025. PMID: 40137251 Free PMC article. Review.
-
Effector Cs02526 from Ciboria shiraiana induces cell death and modulates plant immunity.Plant Physiol. 2024 Sep 2;196(1):579-591. doi: 10.1093/plphys/kiae286. Plant Physiol. 2024. PMID: 38753366 Free PMC article.
-
Type 2C Protein Phosphatase MoPtc6 Plays Critical Roles in the Development and Virulence of Magnaporthe oryzae.J Fungi (Basel). 2025 Apr 24;11(5):335. doi: 10.3390/jof11050335. J Fungi (Basel). 2025. PMID: 40422669 Free PMC article.
-
StRAB4 gene is required for filamentous growth, conidial development, and pathogenicity in Setosphaeria turcica.Front Microbiol. 2024 Jan 8;14:1302081. doi: 10.3389/fmicb.2023.1302081. eCollection 2023. Front Microbiol. 2024. PMID: 38264490 Free PMC article.
-
EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola.J Fungi (Basel). 2025 Apr 9;11(4):295. doi: 10.3390/jof11040295. J Fungi (Basel). 2025. PMID: 40278116 Free PMC article.
References
-
- Andrade-Linares D. R., Veresoglou S. D., Rillig M. C. (2016). Temperature priming and memory in soil filamentous fungi. Fungal Ecol. 21 10–15. 10.1016/j.funeco.2016.02.002 - DOI
LinkOut - more resources
Full Text Sources

