Prevalence, Characteristics and Clonal Distribution of Extended-Spectrum β-Lactamase- and AmpC β-Lactamase-Producing Escherichia coli Following the Swine Production Stages, and Potential Risks to Humans
- PMID: 34367116
- PMCID: PMC8334370
- DOI: 10.3389/fmicb.2021.710747
Prevalence, Characteristics and Clonal Distribution of Extended-Spectrum β-Lactamase- and AmpC β-Lactamase-Producing Escherichia coli Following the Swine Production Stages, and Potential Risks to Humans
Abstract
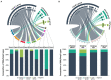
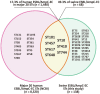
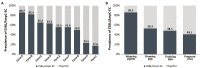
The worldwide spread of extended spectrum β-lactamase (ESBL)- and AmpC β-lactamase (AmpC)-producing Escherichia coli poses serious threats to public health. Swine farms have been regarded as important reservoirs of ESBL/AmpC-EC. This study aimed to determine the prevalence, ESBL/AmpC types, and clonal distribution of ESBL/AmpC-EC from swine farms and analyze the difference according to the swine production stages. In addition, we evaluated the potential risks of swine ESBL/AmpC-EC clones to humans. Individual fecal samples (n = 292) were collected from weaning, growing, finishing, and pregnant pigs in nine swine farms of South Korea between July 2017 and March 2020. In total, 161 ESBL/AmpC-EC isolates were identified (55.1%), with the highest prevalence detected in the weaning stage (86.3%). The dominant ESBL and AmpC types were CTX-M-55 (69.6%) and CMY-2 (4.3%), respectively. CTX-M found in all production stages, while CMY was only found in growing and finishing stages. In the conjugation assay, the high transferability of CTX-M gene (55.8%) was identified, while the transfer of CMY gene was not identified. The major clonal complexes (CCs) were CC101-B1 (26.8%), CC10-A (8.7%), and CC648-F (2.9%). There was similarity in clonal distribution between different swine production stages within swine farms, estimated using the k-means analysis, which suggested a clonal transmission between the different swine stages. Among swine ESBL/AmpC-EC sequence types (STs), seven STs (ST101, ST10, ST648, ST457, ST410, ST617, and ST744) were common with the human ESBL/AmpC-EC, which registered in National Center for Biotechnology Information database. The clonal population structure analysis based on the virulence factor (VF) presented that swine ESBL/AmpC-EC clones, especially ST101-B1, harbored a highly virulent profile. In conclusion, ESBL/AmpC-EC was distributed throughout the swine production stages, with the highest prevalence in the weaning stage. The CTX-M was present in all stages, while CMY was mostly found in growing-finishing stages. The swine ESBL/AmpC-EC was identified to harbor shared clone types with human ESBL/AmpC-EC and a virulent profile posing potential risk to humans. Considering the possibility of genetic and clonal distribution of ESBL/AmpC-EC among swine production stages, this study suggests the need for strategies considering the production system to control the prevalence of ESBL/AmpC-EC in swine farms.
Keywords: AmpC β-lactamase; Escherichia coli; clonal distribution; extended-spectrum β-lactamase; extra-intestinal pathogenic E. coli; multidrug resistance; swine production stages; virulence factor.
Copyright © 2021 Lee, An, Guk, Song, Yi, Kim and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Prevalence, Characteristics, and Clonal Distribution of Escherichia coli Carrying Mobilized Colistin Resistance Gene mcr-1.1 in Swine Farms and Their Differences According to Swine Production Stages.Front Microbiol. 2022 May 6;13:873856. doi: 10.3389/fmicb.2022.873856. eCollection 2022. Front Microbiol. 2022. PMID: 35602044 Free PMC article.
-
Characteristics of Transmissible CTX-M- and CMY-Type β-Lactamase-Producing Escherichia coli Isolates Collected from Pig and Chicken Farms in South Korea.J Microbiol Biotechnol. 2017 Sep 28;27(9):1716-1723. doi: 10.4014/jmb.1610.10006. J Microbiol Biotechnol. 2017. PMID: 28683526
-
Antimicrobial Resistance Genes and Diversity of Clones among ESBL- and Acquired AmpC-Producing Escherichia coli Isolated from Fecal Samples of Healthy and Sick Cats in Portugal.Antibiotics (Basel). 2021 Mar 5;10(3):262. doi: 10.3390/antibiotics10030262. Antibiotics (Basel). 2021. PMID: 33807601 Free PMC article.
-
Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: a threat for humans?Clin Microbiol Infect. 2017 Nov;23(11):826-833. doi: 10.1016/j.cmi.2017.01.013. Epub 2017 Jan 29. Clin Microbiol Infect. 2017. PMID: 28143782 Review.
-
Extended-spectrum β-lactamase-producing Escherichia coli from poultry: A review.Vet World. 2024 Sep;17(9):2017-2027. doi: 10.14202/vetworld.2024.2017-2027. Epub 2024 Sep 8. Vet World. 2024. PMID: 39507773 Free PMC article. Review.
Cited by
-
Distribution of Extended-Spectrum-β-Lactamase-Producing Diarrheagenic Escherichia coli Clonal Complex 10 Isolates from Patients with Diarrhea in the Republic of Korea.Antibiotics (Basel). 2023 Nov 10;12(11):1614. doi: 10.3390/antibiotics12111614. Antibiotics (Basel). 2023. PMID: 37998816 Free PMC article.
-
High prevalence of blaCTX-M-55-carrying Escherichia coli in both ceftiofur-use and non-use pig farms.Appl Environ Microbiol. 2025 Aug 20;91(8):e0252524. doi: 10.1128/aem.02525-24. Epub 2025 Jul 15. Appl Environ Microbiol. 2025. PMID: 40662746 Free PMC article.
-
A Novel CMY Variant Confers Transferable High-Level Resistance to Ceftazidime-Avibactam in Multidrug-Resistant Escherichia coli.Microbiol Spectr. 2023 Feb 14;11(2):e0334922. doi: 10.1128/spectrum.03349-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36786629 Free PMC article.
-
Antimicrobial Resistance in Commensal Escherichia coli of the Porcine Gastrointestinal Tract.Antibiotics (Basel). 2023 Nov 11;12(11):1616. doi: 10.3390/antibiotics12111616. Antibiotics (Basel). 2023. PMID: 37998818 Free PMC article. Review.
-
The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections.Microorganisms. 2022 Aug 31;10(9):1757. doi: 10.3390/microorganisms10091757. Microorganisms. 2022. PMID: 36144359 Free PMC article.
References
-
- Abraham S., Jordan D., Wong H. S., Johnson J. R., Toleman M. A., Wakeham D. L., et al. . (2015). First detection of extended-spectrum cephalosporin- and fluoroquinolone-resistant Escherichia coli in Australian food-producing animals. J. Glob. Antimicrob. Resist. 3, 273–277. 10.1016/j.jgar.2015.08.002, PMID: - DOI - PubMed
-
- Belmahdi M., Bakour S., Al Bayssari C., Touati A., Rolain J. M. (2016). Molecular characterisation of extended-spectrum beta-lactamase- and plasmid AmpC-producing Escherichia coli strains isolated from broilers in Bejaia, Algeria. J. Glob. Antimicrob. Resist. 6, 108–112. 10.1016/j.jgar.2016.04.006, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

