Receptor Tyrosine Kinase Inhibitor Sunitinib as Novel Immunotherapy to Inhibit Myeloid-Derived Suppressor Cells for Treatment of Endometriosis
- PMID: 34367125
- PMCID: PMC8340010
- DOI: 10.3389/fimmu.2021.641206
Receptor Tyrosine Kinase Inhibitor Sunitinib as Novel Immunotherapy to Inhibit Myeloid-Derived Suppressor Cells for Treatment of Endometriosis
Erratum in
-
Correction: Receptor tyrosine kinase inhibitor sunitinib as novel immunotherapy to inhibit myeloid-derived suppressor cells for treatment of endometriosis.Front Immunol. 2025 Nov 24;16:1741271. doi: 10.3389/fimmu.2025.1741271. eCollection 2025. Front Immunol. 2025. PMID: 41368639 Free PMC article.
Abstract
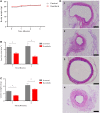
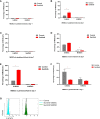
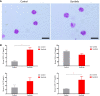
Endometriosis is a common, benign, and hormone-dependent gynaecological disorder that displays altered immunoinflammatory profiles. Myeloid-derived suppressor cells (MDSCs) suppressed immunosurveillance in endometriosis in human and mouse model. Receptor tyrosine kinase inhibitor Sunitinib can induce MDSC apoptosis and suppress the progression of cancer. However, the effects of Sunitinib on MDSCs in endometriosis and the underlying mechanism are not clear. In this study, we employed an animal study of the endometriosis model in mice for treatment of Sunitinib. After syngeneic endometrium transplantation and treatment, endometriotic lesion volume, weight, and histology were compared. Peritoneal fluid, peripheral blood, and bone marrow MDSC subsets and their molecular signaling were monitored by flow cytometry. Peritoneal cytokines were assayed by ELISA. The gene expression profiles of isolated CD11b+Ly6G+Ly6Clo cells were studied by RNA sequencing. We found that Sunitinib significantly decreased the endometriotic lesion size and weight after 1 and 3 weeks, and decreased p-STAT3 activation in MDSCs after 1 week of treatment. In the first week, Sunitinib specifically increased the G-MDSC population in peritoneal fluid but the isolated CD11b+Ly6G+Ly6Clo MDSCs after Sunitinib treatment were presented as mature polynuclear MDSCs, while the control group had immature mononuclear MDSCs. Importantly, we found Sunitinib differentially suppressed gene expressions of immunosuppressive function and differentiation in peritoneal G-MDSCs. Apelin signaling pathway associated genes and inflammation related genes were upregulated, and amino acid metabolism regulator genes were downregulated in bone marrow G-MDSCs. For endometriotic lesions, the PPARG gene governing glucose metabolism and fatty acid storage, which is important for the development of endometriosis was upregulated. In conclusion, Sunitinib inhibited endometriotic lesions, by promoting peritoneal fluid MDSCs maturation and inhibiting the immunosuppressive function. These findings suggest that Sunitinib changed the immune microenvironment and inhibited the development of endometriosis, which has potential therapeutic effects as novel immunotherapy to promote MDSCs maturation, differentiation, and metabolism for the treatment of endometriosis.
Keywords: Sunitinib; endometriosis; gene expressions; immunosuppression; myeloid-derived suppressor cells.
Copyright © 2021 He, Hung, Liang, Zhang, Gao, Chu, Zhang, Xu, Chung and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

