Fecal Bacteria Implicated in Biofilm Production Are Enriched and Associate to Gastrointestinal Symptoms in Patients With APECED - A Pilot Study
- PMID: 34367134
- PMCID: PMC8339580
- DOI: 10.3389/fimmu.2021.668219
Fecal Bacteria Implicated in Biofilm Production Are Enriched and Associate to Gastrointestinal Symptoms in Patients With APECED - A Pilot Study
Abstract
Backgrounds and aims: APECED is a rare autoimmune disease caused by mutations in the Autoimmune Regulator gene. A significant proportion of patients also have gastrointestinal symptoms, including malabsorption, chronic diarrhea, and obstipation. The pathological background of the gastrointestinal symptoms remains incompletely understood and involves multiple factors, with autoimmunity being the most common underlying cause. Patients with APECED have increased immune responses against gut commensals. Our objective was to evaluate whether the intestinal microbiota composition, predicted functions or fungal abundance differ between Finnish patients with APECED and healthy controls, and whether these associate to the patients' clinical phenotype and gastrointestinal symptoms.
Methods: DNA was isolated from fecal samples from 15 patients with APECED (median age 46.4 years) together with 15 samples from body mass index matched healthy controls. DNA samples were subjected to analysis of the gut microbiota using 16S rRNA gene amplicon sequencing, imputed metagenomics using the PICRUSt2 algorithm, and quantitative PCR for fungi. Extensive correlations of the microbiota with patient characteristics were determined.
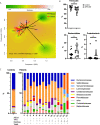
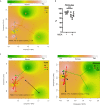
Results: Analysis of gut microbiota indicated that both alpha- and beta-diversity were altered in patients with APECED compared to healthy controls. The fraction of Faecalibacterium was reduced in patients with APECED while that of Atopobium spp. and several gram-negative genera previously implicated in biofilm formation, e.g. Veillonella, Prevotella, Megasphaera and Heamophilus, were increased in parallel to lipopolysaccharide (LPS) synthesis in imputed metagenomics. The differences in gut microbiota were linked to patient characteristics, especially the presence of anti-Saccharomyces cerevisiae antibodies (ASCA) and severity of gastrointestinal symptoms.
Conclusions: Gut microbiota of patients with APECED is altered and enriched with predominantly gram-negative bacterial taxa that may promote biofilm formation and lead to increased exposure to LPS in the patients. The most pronounced alterations in the microbiota were associated with more severe gastrointestinal symptoms.
Keywords: atopobium; autoantibodies; autoimmunity; dysbiosis; faecalibacterium; gut microbiota; immune dysregulation; lps.
Copyright © 2021 Hetemäki, Jian, Laakso, Mäkitie, Pajari, de Vos, Arstila and Salonen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

