Allotransplantation Is Associated With Exacerbation of CD8 T-Cell Senescence: The Particular Place of the Innate CD8 T-Cell Component
- PMID: 34367138
- PMCID: PMC8334557
- DOI: 10.3389/fimmu.2021.674016
Allotransplantation Is Associated With Exacerbation of CD8 T-Cell Senescence: The Particular Place of the Innate CD8 T-Cell Component
Abstract
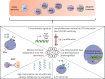
Immunosenescence is a physiological process that is associated with changes in the immune system, particularly among CD8 T-cells. Recent studies have hypothesized that senescent CD8 T-cells are produced with chronologic age by chronic stimulation, leading to the acquisition of hallmarks of innate-like T-cells. While conventional CD8 T-cells are quite well characterized, CD8 T-cells sharing features of NK cells and memory CD8 T-cells, are a newly described immune cell population. They can be distinguished from conventional CD8 T-cells by their combined expression of panKIR/NKG2A and Eomesodermin (E), a unique phenotype closely associated with IFN-γ production in response to innate stimulation. Here, we first provided new evidence in favor of the innate character of panKIR/NKG2A(+) E(+) CD8 T-cells in normal subjects, documenting their position at an intermediate level in the innateness gradient in terms of both innate IFN-γ production and diminished mitochondrial mass. We also revealed that CD8 E(+) panKIR/NKG2A(+) T-cells, hereafter referred to as Innate E(+) CD8 T-cells, exhibit increased senescent (CD27(-) CD28(-)) phenotype, compared to their conventional memory counterparts. Surprisingly, this phenomenon was not dependent on age. Given that inflammation related to chronic viral infection is known to induce NK-like marker expression and a senescence phenotype among CD8 T-cells, we hypothesized that innate E(+) CD8 T-cells will be preferentially associated with exacerbated cellular senescence in response to chronic alloantigen exposure or CMV infection. Accordingly, in a pilot cohort of stable kidney allotransplant recipients, we observed an increased frequency of the Innate E(+) CD8 T-cell subset, together with an exacerbated senescent phenotype. Importantly, this phenotype cannot be explained by age alone, in clear contrast to their conventional memory counterparts. The senescent phenotype in CD8 T-cells was further increased in cytomegalovirus (CMV) positive serology transplant recipients, suggesting that transplantation and CMV, rather than aging by itself, may promote an exacerbated senescent phenotype of innate CD8 T-cells. In conclusion, we proposed that kidney transplantation, via the setting of inflammatory stimuli of alloantigen exposure and CMV infection, may exogenously age the CD8 T-cell compartment, especially its innate component. The physiopathological consequences of this change in the immune system remain to be elucidated.
Keywords: NK-like T-cells; allotransplantation; immune memory; innate memory CD8(+) T-cells; innateness gradient; senescence.
Copyright © 2021 Daniel, Tassery, Lateur, Thierry, Herbelin, Gombert and Barbarin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

