Exosome-Depleted Excretory-Secretory Products of the Fourth-Stage Larval Angiostrongylus cantonensis Promotes Alternative Activation of Macrophages Through Metabolic Reprogramming by the PI3K-Akt Pathway
- PMID: 34367145
- PMCID: PMC8343011
- DOI: 10.3389/fimmu.2021.685984
Exosome-Depleted Excretory-Secretory Products of the Fourth-Stage Larval Angiostrongylus cantonensis Promotes Alternative Activation of Macrophages Through Metabolic Reprogramming by the PI3K-Akt Pathway
Abstract
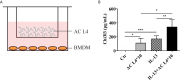
Angiostrongylus cantonensis (AC), which parasitizes in the brain of the non-permissive host, such as mouse and human, is an etiologic agent of eosinophilic meningitis. Excretory-secretory (ES) products play an important role in the interaction between parasites and hosts' immune responses. Inflammatory macrophages are responsible for eosinophilic meningitis induced by AC, and the soluble antigens of Angiostrongylus cantonensis fourth stage larva (AC L4), a mimic of dead AC L4, aggravate eosinophilic meningitis in AC-infected mice model via promoting alternative activation of macrophages. In this study, we investigated the key molecules in the ES products of AC L4 on macrophages and observed the relationship between metabolic reprogramming and the PI3K-Akt pathway. First, a co-culture system of macrophage and AC L4 was established to define the role of AC L4 ES products on macrophage polarization. Then, AC L4 exosome and exosome-depleted excretory-secretory products (exofree) were separated from AC L4 ES products using differential centrifugation, and their distinct roles on macrophage polarization were confirmed using qPCR and ELISA experiments. Moreover, AC L4 exofree induced alternative activation of macrophages, which is partially associated with metabolic reprogramming by the PI3K-Akt pathway. Next, lectin blot and deglycosylation assay were done, suggesting the key role of N-linked glycoproteins in exofree. Then, glycoproteomic analysis of exofree and RNA-seq analysis of exofree-treated macrophage were performed. Bi-layer PPI network analysis based on these results identified macrophage-related protein Hexa as a key molecule in inducing alternative activation of macrophages. Our results indicate a great value for research of helminth-derived immunoregulatory molecules, which might contribute to drug development for immune-related diseases.
Keywords: Angiostrongylus cantonensis; N-linked glycoproteins; exosome-depleted excretory-secretory products; macrophage polarization; mechanism.
Copyright © 2021 Wan, Sun, Tang, Wang, Wu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Prasanphanich NS, Luyai AE, Song X, Heimburg-Molinaro J, Mandalasi M, Mickum M, et al. Immunization With Recombinantly Expressed Glycan Antigens From Schistosoma Mansoni Induces Glycan-Specific Antibodies Against the Parasite. Glycobiology (2014) 24:619–37. 10.1093/glycob/cwu027 - DOI - PMC - PubMed
-
- Eichenberger RM, Talukder MH, Field MA, Wangchuk P, Giacomin P, Loukas A, et al. Characterization of Trichuris Muris Secreted Proteins and Extracellular Vesicles Provides New Insights Into Host-Parasite Communication. J Extracell Vesicles (2018) 7:1428004. 10.1080/20013078.2018.1428004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

