S100A4 Is Critical for a Mouse Model of Allergic Asthma by Impacting Mast Cell Activation
- PMID: 34367151
- PMCID: PMC8341765
- DOI: 10.3389/fimmu.2021.692733
S100A4 Is Critical for a Mouse Model of Allergic Asthma by Impacting Mast Cell Activation
Abstract
Background: The calcium-binding protein S100A4 demonstrates important regulatory roles in many biological processes including tumorigenesis and inflammatory disorders such as allergy. However, the specific mechanism of the contribution of S100A4 to allergic diseases awaits further clarification.
Objective: To address the effect of S100A4 on the regulation of mast cell activation and its impact on allergy.
Methods: Bone marrow-derived cultured mast cells (BMMCs) were derived from wild-type (WT) or S100A4-/- mice for in vitro investigation. WT and S100A4-/- mice were induced to develop a passive cutaneous anaphylaxis (PCA) model, a passive systemic anaphylaxis (PSA) model, and an ovalbumin (OVA)-mediated mouse asthma model.
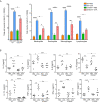
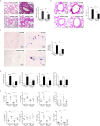
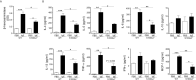
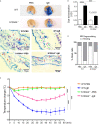
Results: Following OVA/alum-based sensitization and provocation, S100A4-/- mice demonstrated overall suppressed levels of serum anti-OVA IgE and IgG antibodies and proinflammatory cytokines in serum, bronchoalveolar lavage fluid (BALF), and lung exudates. S100A4-/- mice exhibited less severe asthma signs which included inflammatory cell infiltration in the lung tissue and BALF, and suppressed mast cell recruitment in the lungs. Reduced levels of antigen reencounter-induced splenocyte proliferation in vitro were recorded in splenocytes from OVA-sensitized and challenged mice that lacked S100A4-/-. Furthermore, deficiency in the S100A4 gene could dampen mast cell activation both in vitro and in vivo, evidenced by reduced β-hexosaminidase release and compromised PCA and PSA reaction. We also provided evidence supporting the expression of S100A4 by mast cells.
Conclusion: S100A4 is required for mast cell functional activation, and S100A4 may participate in the regulation of allergic responses at least partly through regulating the activation of mast cells.
Keywords: S100A4; airway inflammation; allergic asthma; allergy; mast cell.
Copyright © 2021 Wu, Ma, Jin, He, Chen, Zhang, Yuan, Yang, Zhong, Zhou, Xiang and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

