TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer
- PMID: 34367161
- PMCID: PMC8339559
- DOI: 10.3389/fimmu.2021.699895
TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer
Abstract
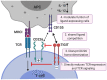
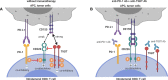
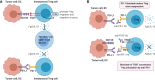
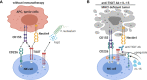
T cell immunoreceptor with Ig and ITIM domains (TIGIT) is an inhibitory receptor expressed on several types of lymphocytes. Efficacy of antibody blockade of TIGIT in cancer immunotherapy is currently widely being investigated in both pre-clinical and clinical studies. In multiple cancers TIGIT is expressed on tumor-infiltrating cytotoxic T cells, helper T cells, regulatory T cells and NK cells, and its main ligand CD155 is expressed on tumor-infiltrating myeloid cells and upregulated on cancer cells, which contributes to local suppression of immune-surveillance. While single TIGIT blockade has limited anti-tumor efficacy, pre-clinical studies indicate that co-blockade of TIGIT and PD-1/PD-L1 pathway leads to tumor rejection, notably even in anti-PD-1 resistant tumor models. Among inhibitory immune checkpoint molecules, a unique property of TIGIT blockade is that it enhances not only anti-tumor effector T-cell responses, but also NK-cell responses, and reduces the suppressive capacity of regulatory T cells. Numerous clinical trials on TIGIT-blockade in cancer have recently been initiated, predominantly combination treatments. The first interim results show promise for combined TIGIT and PD-L1 co-blockade in solid cancer patients. In this review, we summarize the current knowledge and identify the gaps in our current understanding of TIGIT's roles in cancer immunity, and provide, based on these insights, recommendations for its positioning in cancer immunotherapy.
Keywords: CD155; CD226; NK cells; T cells; immunotherapy.
Copyright © 2021 Ge, Peppelenbosch, Sprengers and Kwekkeboom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in Patients With Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet (2017) 389:2492–502. 10.1016/S0140-6736(17)31046-2 - DOI - PMC - PubMed
-
- Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH, Jr., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol (2018) 36:383–90. 10.1200/JCO.2016.71.8023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

