Adenovirus Armed With TNFa and IL2 Added to aPD-1 Regimen Mediates Antitumor Efficacy in Tumors Refractory to aPD-1
- PMID: 34367166
- PMCID: PMC8343222
- DOI: 10.3389/fimmu.2021.706517
Adenovirus Armed With TNFa and IL2 Added to aPD-1 Regimen Mediates Antitumor Efficacy in Tumors Refractory to aPD-1
Abstract
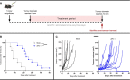
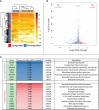
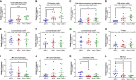
Immune checkpoint inhibitors such as anti-PD-1 have revolutionized the field of oncology over the past decade. Nevertheless, the majority of patients do not benefit from them. Virotherapy is a flexible tool that can be used to stimulate and/or recruit different immune populations. T-cell enabling virotherapy could enhance the efficacy of immune checkpoint inhibitors, even in tumors resistant to these inhibitors. The T-cell potentiating virotherapy used here consisted of adenoviruses engineered to express tumor necrosis factor alpha and interleukin-2 in the tumor microenvironment. To study virus efficacy in checkpoint-inhibitor resistant tumors, we developed an anti-PD-1 resistant melanoma model in vivo. In resistant tumors, adding virotherapy to an anti-PD-1 regimen resulted in increased survival (p=0.0009), when compared to anti-PD-1 monotherapy. Some of the animals receiving virotherapy displayed complete responses, which did not occur in the immune checkpoint-inhibitor monotherapy group. When adenoviruses were delivered into resistant tumors, there were signs of increased CD8 T-cell infiltration and activation, which - together with a reduced presence of M2 macrophages and myeloid-derived suppressor cells - could explain those results. T-cell enabling virotherapy appeared as a valuable tool to counter resistance to immune checkpoint inhibitors. The clinical translation of this approach could increase the number of cancer patients benefiting from immunotherapies.
Keywords: adenovirus; cancer immunotherapy; checkpoint inhibitor resistance; oncolytic virus; tumor microenvironment.
Copyright © 2021 Cervera-Carrascon, Quixabeira, Santos, Havunen, Milenova, Verhoeff, Heiniö, Zafar, Garcia-Vallejo, van Beusechem, de Gruijl, Kalervo, Sorsa, Kanerva and Hemminki.
Conflict of interest statement
AH is shareholder in Targovax ASA (Oslo, Norway) and in TILT Biotherapeutics Ltd. (Helsinki, Finland). AH, RH, SS, JS, and VC-C are employees of TILT Biotherapeutics Ltd. IM is employed by Orca Therapeutics, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

