Cytochrome P450 Enzymes as Key Drivers of Alkaloid Chemical Diversification in Plants
- PMID: 34367208
- PMCID: PMC8336426
- DOI: 10.3389/fpls.2021.682181
Cytochrome P450 Enzymes as Key Drivers of Alkaloid Chemical Diversification in Plants
Abstract
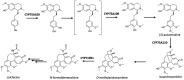
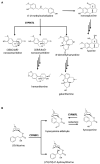
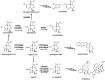
Plants produce more than 20,000 nitrogen-containing heterocyclic metabolites called alkaloids. These chemicals serve numerous eco-physiological functions in the plants as well as medicines and psychedelic drugs for human for thousands of years, with the anti-cancer agent vinblastine and the painkiller morphine as the best-known examples. Cytochrome P450 monooxygenases (P450s) play a key role in generating the structural variety that underlies this functional diversity of alkaloids. Most alkaloid molecules are heavily oxygenated thanks to P450 enzymes' activities. Moreover, the formation and re-arrangement of alkaloid scaffolds such as ring formation, expansion, and breakage that contribute to their structural diversity and bioactivity are mainly catalyzed by P450s. The fast-expanding genomics and transcriptomics databases of plants have accelerated the investigation of alkaloid metabolism and many players behind the complexity and uniqueness of alkaloid biosynthetic pathways. Here we discuss recent discoveries of P450s involved in the chemical diversification of alkaloids and how these inform our approaches in understanding plant evolution and producing plant-derived drugs.
Keywords: P450; alkaloid; catalysis; diversification; medicinal plants; oxidation; scaffold.
Copyright © 2021 Nguyen and Dang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Benayad S., Ahamada K., Lewin G., Evanno L., Poupon E. (2016). Preakuammicine: a long-awaited missing link in the biosynthesis of monoterpene indole alkaloids. Eur. J. Org. Chem. 2016, 1494–1499. 10.1002/ejoc.201600102 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

