A Novel Expression Signature from the Perspective of Mesenchymal-Epithelial Transition for Hepatocellular Carcinoma with Regard to Prognosis, Clinicopathological Features, Immune Cell Infiltration, Chemotherapeutic Efficacy, and Immunosuppressive Molecules
- PMID: 34367283
- PMCID: PMC8342179
- DOI: 10.1155/2021/5033416
A Novel Expression Signature from the Perspective of Mesenchymal-Epithelial Transition for Hepatocellular Carcinoma with Regard to Prognosis, Clinicopathological Features, Immune Cell Infiltration, Chemotherapeutic Efficacy, and Immunosuppressive Molecules
Abstract
Purpose: Mesenchymal-epithelial transition (MET), a reverse biological process to epithelial-mesenchymal transition (EMT), is involved in tumor metastasis and invasion. However, the role of MET-related genes (MRGs) in hepatocellular carcinoma (HCC) prognosis remains unclear.
Methods: In this research, we obtained MRGs data and clinical information from public databases. In the TCGA dataset, a prognostic signature for HCC was constructed by the least absolute shrinkage and selection operator (LASSO) method and externally verified using the ICGC dataset.
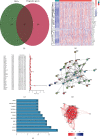
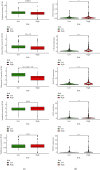
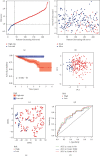
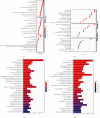
Results: There were 148 differentially expressed MRGs (DEMRGs), out of which 37 MRGs were found associated with overall survival (OS) in the univariate Cox analysis. A novel signature integrating of 5 MRGs was constructed, which split patients into high- and low-risk groups. Kaplan-Meier analysis revealed that high-risk patients had unfavorable OS than those low-risk counterparts. Receiver operating characteristic curve (ROC) showed great performance of this signature in predictive ability. Multivariate Cox analysis confirmed that this signature could independently predict HCC prognosis. The analysis of immune cell infiltration demonstrated that immune status varied differently between high- and low-risk groups. The analysis of clinicopathological characteristics suggested that tumor grade, clinical stage, and T stage were different between risk groups. The analysis between this signature and chemotherapeutic efficacy and immunosuppressive molecules indicated that this signature could serve as a promising predictor.
Conclusions: In conclusion, we constructed and verified a novel signature from the perspective of MET, which was significantly associated with HCC prognosis, clinicopathological features, immune status, chemotherapeutic efficacy, and immunosuppressive biomarkers.
Copyright © 2021 Lijun Xu and Qing Zheng.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

