Memory retention in pyramidal neurons: a unified model of energy-based homo and heterosynaptic plasticity with homeostasis
- PMID: 34367368
- PMCID: PMC8286921
- DOI: 10.1007/s11571-020-09652-z
Memory retention in pyramidal neurons: a unified model of energy-based homo and heterosynaptic plasticity with homeostasis
Abstract
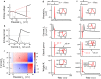
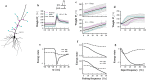
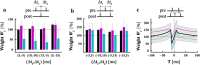
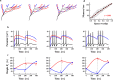
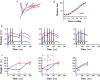
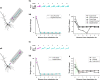
The brain can learn new tasks without forgetting old ones. This memory retention is closely associated with the long-term stability of synaptic strength. To understand the capacity of pyramidal neurons to preserve memory under different tasks, we established a plasticity model based on the postsynaptic membrane energy state, in which the change in synaptic strength depends on the difference between the energy state after stimulation and the resting energy state. If the post-stimulation energy state is higher than the resting energy state, then synaptic depression occurs. On the contrary, the synapse is strengthened. Our model unifies homo- and heterosynaptic plasticity and can reproduce synaptic plasticity observed in multiple experiments, such as spike-timing-dependent plasticity, and cooperative plasticity with few and common parameters. Based on the proposed plasticity model, we conducted a simulation study on how the activation patterns of dendritic branches by different tasks affect the synaptic connection strength of pyramidal neurons. We further investigate the formation mechanism by which different tasks activate different dendritic branches. Simulation results show that compare to the classic plasticity model, the plasticity model we proposed can achieve a better spatial separation of different branches activated by different tasks in pyramidal neurons, which deepens our insight into the memory retention mechanism of brains.
Keywords: Homo- and heterosynaptic plasticity; Memory retention; Metabolic energy; Neural computation; Pyramidal neuron.
© Springer Nature B.V. 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures


Similar articles
-
Partial Breakdown of Input Specificity of STDP at Individual Synapses Promotes New Learning.J Neurosci. 2016 Aug 24;36(34):8842-55. doi: 10.1523/JNEUROSCI.0552-16.2016. J Neurosci. 2016. PMID: 27559167 Free PMC article.
-
Heterosynaptic plasticity prevents runaway synaptic dynamics.J Neurosci. 2013 Oct 2;33(40):15915-29. doi: 10.1523/JNEUROSCI.5088-12.2013. J Neurosci. 2013. PMID: 24089497 Free PMC article.
-
Postsynaptic Potential Energy as Determinant of Synaptic Plasticity.Front Comput Neurosci. 2022 Feb 17;16:804604. doi: 10.3389/fncom.2022.804604. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35250524 Free PMC article.
-
Heterosynaptic plasticity in the neocortex.Exp Brain Res. 2009 Dec;199(3-4):377-90. doi: 10.1007/s00221-009-1859-5. Exp Brain Res. 2009. PMID: 19499213 Free PMC article. Review.
-
'Synaptic tagging' and 'cross-tagging' and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation.Prog Brain Res. 2008;169:117-43. doi: 10.1016/S0079-6123(07)00007-6. Prog Brain Res. 2008. PMID: 18394471 Review.
References
-
- Abraham WC, Logan B, Wolff A, Benuskova L. Heterosynaptic. LTD in the dentate gyrus of anesthetized rat requires homosynaptic activity, J Neurophysiol. 2007;98(2):1048–1051. - PubMed
LinkOut - more resources
Full Text Sources

