Interleukin-18: A Novel Participant in the Occurrence, Development, and Drug Therapy of Obliterative Bronchiolitis Postlung Transplantation
- PMID: 34367377
- PMCID: PMC8337162
- DOI: 10.1155/2021/5586312
Interleukin-18: A Novel Participant in the Occurrence, Development, and Drug Therapy of Obliterative Bronchiolitis Postlung Transplantation
Abstract
Background: Obliterative bronchiolitis (OB) was a main cause of deterioration of long-term prognosis in lung transplant recipients after the first posttransplant year. Proinflammatory cytokine interleukin-18 (IL-18) strengthened both the natural immunity and acquired immunity and played an important role in organ transplantation. The roles of IL-18 in the occurrence, development, and drug treatment of OB remained unclear.
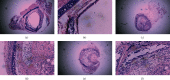
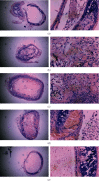
Methods: Small interfering RNA (siRNA) against mouse IL-18 (siRNA-IL-18) was used to silence IL-18 expression. Mouse heterotopic tracheal transplantation model was used to simulate OB. Recipient mice were divided into 5 groups (n = 12) according to donor mouse strains and drug treatment: isograft group, allograft group, allograft+tacrolimus group, allograft+azithromycin group, and allograft+tacrolimus+azithromycin group. The luminal obliteration rates were pathological evaluation. Expressions of cytokines and MMPs were detected by real-time PCR, western blot, and enzyme chain immunosorbent assay (ELISA).
Results: The luminal obliteration rates of IL-18 of the siRNA-IL-18 group were significantly lower than those of the negative control group (p < 0.0001) and the blank control group (p = 0.0002). mRNA expressions of IFN-γ, EMMPRIN, MMP-8, and MMP-9 of the siRNA-IL-18 group were significantly lower than those of the negative and blank control groups. No tracheal occlusion occurred in grafts of the isograft group. The rates of tracheal occlusion of the allograft group, allograft+tacrolimus group, allograft+azithromycin group, and allograft+tacrolimus+azithromycin group were 72.17 ± 4.66%, 40.33 ± 3.00%, 38.50 ± 2.08%, and 23.33 ± 3.24%, respectively. There were significant differences between the 4 groups (p < 0.001). Serum protein expressions of IL-17 (p = 0.0017), IL-18 (p = 0.0036), IFN-γ (p = 0.0102), and MMP-9 (p = 0.0194) were significantly decreased in the allograft+tacrolimus+azithromycin group compared to the allograft group.
Conclusions: IL-18 could be a novel molecular involved in the occurrence, development, and drug treatment of OB.
Copyright © 2021 Ping Shu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
FK506 combined with GM6001 prevents tracheal obliteration in a mouse model of heterotopic tracheal transplantation.Transpl Immunol. 2019 Dec;57:101244. doi: 10.1016/j.trim.2019.101244. Epub 2019 Sep 14. Transpl Immunol. 2019. PMID: 31526865
-
[Establishment of obliterative bronchiolitis in allo-trachea transplant model of rat and detection of its pathogenesis preliminarily].Zhonghua Wai Ke Za Zhi. 2007 Feb 15;45(4):262-6. Zhonghua Wai Ke Za Zhi. 2007. PMID: 17502025 Chinese.
-
Azithromycin Fails to Prevent Accelerated Airway Obliteration in T-bet-/- Mouse Lung Allograft Recipients.Transplant Proc. 2018 Jun;50(5):1566-1574. doi: 10.1016/j.transproceed.2018.02.070. Epub 2018 Mar 9. Transplant Proc. 2018. PMID: 29880387 Free PMC article.
-
A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy.Eur Respir J. 2008 Oct;32(4):832-43. doi: 10.1183/09031936.00134307. Eur Respir J. 2008. PMID: 18827151 Review.
-
Chronic lung allograft dysfunction after lung transplantation: the moving target.Gen Thorac Cardiovasc Surg. 2013 Feb;61(2):67-78. doi: 10.1007/s11748-012-0167-3. Epub 2012 Nov 10. Gen Thorac Cardiovasc Surg. 2013. PMID: 23138970 Review.
References
-
- Arjuna A., Olson M. T., Walia R., Bremner R. M., Smith M. A., Mohanakumar T. An update on current treatment strategies for managing bronchiolitis obliterans syndrome after lung transplantation. Expert Review of Respiratory Medicine. 2021;15(3):339–350. doi: 10.1080/17476348.2021.1835475. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

