Patiromer and Sodium Zirconium Cyclosilicate in Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis
- PMID: 34367383
- PMCID: PMC8326359
- DOI: 10.1016/j.curtheres.2021.100635
Patiromer and Sodium Zirconium Cyclosilicate in Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis
Abstract
Background: Patiromer and sodium zirconium cyclosilicate (SZC) are newer options for hyperkalemia treatment. This systematic review and meta-analysis were conducted to assess the safety and side effect profile of patiromer and SZC compared with placebo or other standards of care in the management of hyperkalemia.
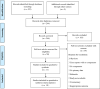
Methods: We searched electronic databases for relevant articles. The screening was performed independently and data were extracted among the selected studies. We performed a statistical analysis on Revman 5.4 software. The odds ratio (OR) was used for outcome estimation with a 95% CI.
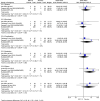
Results: Patiromer had lower rates of hyperkalemia (OR = 0.44; 95% CI, 0.22-0.89) compared with standard of care. The analysis showed no significant differences between the 2 groups in terms of overall adverse effects, any serious/specific adverse effects, or treatment discontinuation as a result of adverse effects. Comparing the SZC-10 group with standard of care showed no significant differences in the occurrence of hyperkalemia during treatment, overall adverse effects, any serious/specific adverse effects, or treatment discontinuation as a result of adverse effects but showed a higher rate of edema in the treatment group (OR = 6.77; 95% CI, 1.03-44.25). Similarly, no significant differences were seen between the 2 SZC doses for the occurrence of any adverse effects, hyperkalemia, constipation, diarrhea, or urinary tract infection, whereas edema was higher among patients receiving SZC-10 (OR = 3.13; 95% CI, 1.19-8.27).
Conclusions: In patients with acute hyperkalemia, SZC is the drug of choice due to its more rapid reduction of serum potassium level, whereas in patients with chronic hyperkalemia, patiromer appears to be the drug of choice because SZC is associated with an increase in edema, likely due to an increase in sodium absorption, which could have important adverse consequences in patients with chronic kidney disease and or heart failure. Thus, both drugs were found to be safe while treating hyperkalemia. (Curr Ther Res Clin Exp. 2021; 82:XXX-XXX).
Keywords: hyperkalemia; patiromer; potassium; sodium zirconium cyclosilicate.
© 2021 The Author(s).
Figures




References
-
- Veltassa (patiromer) FDA Approval History - Drugs.com. Accessed December 18, 2020. https://www.drugs.com/history/veltassa.html
Publication types
LinkOut - more resources
Full Text Sources

