Enhanced Sensitivity of Nonsmall Cell Lung Cancer with Acquired Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors to Phenformin: The Roles of a Metabolic Shift to Oxidative Phosphorylation and Redox Balance
- PMID: 34367462
- PMCID: PMC8342158
- DOI: 10.1155/2021/5428364
Enhanced Sensitivity of Nonsmall Cell Lung Cancer with Acquired Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors to Phenformin: The Roles of a Metabolic Shift to Oxidative Phosphorylation and Redox Balance
Abstract
Background: Although the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR- TKI) therapy has been proven in non-small cell lung cancer (NSCLC) patients, acquired resistance to EGFR-TKIs presents a serious clinical problem. Hence, the identification of new therapeutic strategy is needed to treat EGFR-TKI-resistant NSCLC.
Methods: Acquired EGFR-TKI-resistant lung cancer cell lines (HCC827, H1993, and H292 cells with acquired resistance to gefitinib or erlotinib) were used for cell-based studies. IncuCyte live cell analysis system and XFp analyzer were used for the determination of cell proliferation and energy metabolism, respectively. In vivo anticancer effect of phenformin was assessed in xenografts implanting HCC827 and gefitinib-resistant HCC827 (HCC827 GR) cells.
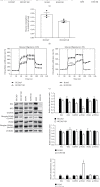
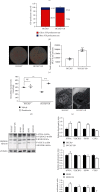
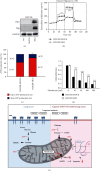
Results: HCC827 GR and erlotinib-resistant H1993 (H1993 ER) cells exhibited different metabolic properties compared with their respective parental cells, HCC827, and H1993. In EGFR-TKI-resistant NSCLC cells, glycolysis markers including the glucose consumption rate, intracellular lactate level, and extracellular acidification rate were decreased; however, mitochondrial oxidative phosphorylation (OXPHOS) markers including mitochondria-driven ATP production, mitochondrial membrane potential, and maximal OXPHOS capacity were increased. Cell proliferation and tumor growth were strongly inhibited by biguanide phenformin via targeting of mitochondrial OXPHOS complex 1 in EGFR-TKI-resistant NSCLC cells. Inhibition of OXPHOS resulted in a reduced NAD+/NADH ratio and intracellular aspartate levels. Recovery of glycolysis by hexokinase 2 overexpression in erlotinib-resistant H292 (H292 ER) cells significantly reduced the anticancer effects of phenformin.
Conclusion: Long-term treatment with EGFR-TKIs causes reactivation of mitochondrial metabolism, resulting in vulnerability to OXPHOS inhibitor such as phenformin. We propose a new therapeutic option for NSCLC with acquired EGFR-TKI resistance that focuses on cancer metabolism.
Copyright © 2021 Suntae Kim et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- FDA resources for Information. Food and Drug Administration Web site. 2018, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

