Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair
- PMID: 34367479
- PMCID: PMC8316866
- DOI: 10.4252/wjsc.v13.i7.825
Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair
Abstract
Osteoarthritis (OA) is the most prevalent joint disease causing major disability and medical expenditures. Synovitis is a central feature of OA and is primarily driven by macrophages. Synovial macrophages not only drive inflammation but also its resolution, through a coordinated, simultaneous expression of pro- and anti-inflammatory mechanisms that are essential to counteract damage and recover homeostasis. Current OA therapies are largely based on anti-inflammatory principles and therefore block pro-inflammatory mechanisms such as prostaglandin E2 and Nuclear factor-kappa B signaling pathways. However, such mechanisms are also innately required for mounting a pro-resolving response, and their blockage often results in chronic low-grade inflammation. Following minor injury, macrophages shield the damaged area and drive tissue repair. If the damage is more extensive, macrophages incite inflammation recruiting more macrophages from the bone marrow to maximize tissue repair and ultimately resolve inflammation. However, sustained damage and inflammation often overwhelms pro-resolving mechanisms of synovial macrophages leading to the chronic inflammation and related tissue degeneration observed in OA. Recently, experimental and clinical studies have shown that joint injection with autologous bone marrow mononuclear cells replenishes inflamed joints with macrophage and hematopoietic progenitors, enhancing mechanisms of inflammation resolution, providing remarkable and long-lasting effects. Besides creating an ideal environment for resolution with high concentrations of interleukin-10 and anabolic growth factors, macrophage progenitors also have a direct role in tissue repair. Macrophages constitute a large part of the early granulation tissue, and further transdifferentiate from myeloid into a mesenchymal phenotype. These cells, characterized as fibrocytes, are essential for repairing osteochondral defects. Ongoing "omics" studies focused on identifying key drivers of macrophage-mediated resolution of joint inflammation and those required for efficient osteochondral repair, have the potential to uncover ways for developing engineered macrophages or off-the-shelf pro-resolving therapies that can benefit patients suffering from many types of arthropaties, not only OA.
Keywords: Arthropathy; Cell therapy; Hematopoietic progenitor; Homeostasis; Osteoarthritis; Synovitis.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: We have no conflicts of interest to declare.
Figures

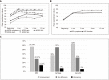

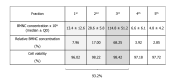
Similar articles
-
Transcriptional and Histochemical Signatures of Bone Marrow Mononuclear Cell-Mediated Resolution of Synovitis.Front Immunol. 2021 Dec 8;12:734322. doi: 10.3389/fimmu.2021.734322. eCollection 2021. Front Immunol. 2021. PMID: 34956173 Free PMC article.
-
Autologous bone marrow mononuclear cells modulate joint homeostasis in an equine in vivo model of synovitis.FASEB J. 2019 Dec;33(12):14337-14353. doi: 10.1096/fj.201901684RR. Epub 2019 Oct 30. FASEB J. 2019. PMID: 31665925
-
Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy.FASEB J. 2020 Mar;34(3):4430-4444. doi: 10.1096/fj.201902698R. Epub 2020 Feb 6. FASEB J. 2020. PMID: 32030831
-
Immunoregulation of synovial macrophages for the treatment of osteoarthritis.Open Life Sci. 2023 Jan 31;18(1):20220567. doi: 10.1515/biol-2022-0567. eCollection 2023. Open Life Sci. 2023. PMID: 36789002 Free PMC article. Review.
-
Role of Mesenchymal Stem Cells and Their Paracrine Mediators in Macrophage Polarization: An Approach to Reduce Inflammation in Osteoarthritis.Int J Mol Sci. 2022 Oct 27;23(21):13016. doi: 10.3390/ijms232113016. Int J Mol Sci. 2022. PMID: 36361805 Free PMC article. Review.
Cited by
-
M2 Macrophage-Derived Extracellular Vehicles-Loaded Hyaluronic Acid-Alginate Hydrogel for Treatment of Osteoarthritis.Orthop Surg. 2025 Jun;17(6):1867-1881. doi: 10.1111/os.70059. Epub 2025 May 13. Orthop Surg. 2025. PMID: 40358119 Free PMC article.
-
Intra-articular bone marrow mononuclear cell therapy improves lameness from naturally occurring equine osteoarthritis.Front Vet Sci. 2023 Oct 9;10:1256284. doi: 10.3389/fvets.2023.1256284. eCollection 2023. Front Vet Sci. 2023. PMID: 37876630 Free PMC article.
-
Magnetic nanomagnetic nanoparticles combining with Slit2 gene and bone marrow mononuclear cells to improve cognitive dysfunction in rats with chronic cerebral ischemia.Int J Med Sci. 2024 Aug 19;21(11):2233-2243. doi: 10.7150/ijms.97051. eCollection 2024. Int J Med Sci. 2024. PMID: 39239546 Free PMC article.
-
Osteochondral organoids: current advances, applications, and upcoming challenges.Stem Cell Res Ther. 2024 Jun 21;15(1):183. doi: 10.1186/s13287-024-03790-5. Stem Cell Res Ther. 2024. PMID: 38902814 Free PMC article. Review.
References
-
- Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res (Hoboken) . 2018;70:869–876. - PubMed
-
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol . 2010;6:625–635. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

