Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair
- PMID: 34367479
- PMCID: PMC8316866
- DOI: 10.4252/wjsc.v13.i7.825
Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair
Abstract
Osteoarthritis (OA) is the most prevalent joint disease causing major disability and medical expenditures. Synovitis is a central feature of OA and is primarily driven by macrophages. Synovial macrophages not only drive inflammation but also its resolution, through a coordinated, simultaneous expression of pro- and anti-inflammatory mechanisms that are essential to counteract damage and recover homeostasis. Current OA therapies are largely based on anti-inflammatory principles and therefore block pro-inflammatory mechanisms such as prostaglandin E2 and Nuclear factor-kappa B signaling pathways. However, such mechanisms are also innately required for mounting a pro-resolving response, and their blockage often results in chronic low-grade inflammation. Following minor injury, macrophages shield the damaged area and drive tissue repair. If the damage is more extensive, macrophages incite inflammation recruiting more macrophages from the bone marrow to maximize tissue repair and ultimately resolve inflammation. However, sustained damage and inflammation often overwhelms pro-resolving mechanisms of synovial macrophages leading to the chronic inflammation and related tissue degeneration observed in OA. Recently, experimental and clinical studies have shown that joint injection with autologous bone marrow mononuclear cells replenishes inflamed joints with macrophage and hematopoietic progenitors, enhancing mechanisms of inflammation resolution, providing remarkable and long-lasting effects. Besides creating an ideal environment for resolution with high concentrations of interleukin-10 and anabolic growth factors, macrophage progenitors also have a direct role in tissue repair. Macrophages constitute a large part of the early granulation tissue, and further transdifferentiate from myeloid into a mesenchymal phenotype. These cells, characterized as fibrocytes, are essential for repairing osteochondral defects. Ongoing "omics" studies focused on identifying key drivers of macrophage-mediated resolution of joint inflammation and those required for efficient osteochondral repair, have the potential to uncover ways for developing engineered macrophages or off-the-shelf pro-resolving therapies that can benefit patients suffering from many types of arthropaties, not only OA.
Keywords: Arthropathy; Cell therapy; Hematopoietic progenitor; Homeostasis; Osteoarthritis; Synovitis.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: We have no conflicts of interest to declare.
Figures

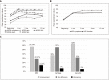

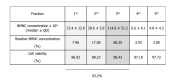
References
-
- Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res (Hoboken) . 2018;70:869–876. - PubMed
-
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol . 2010;6:625–635. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

