Stem cell therapy: A paradigm shift in breast cancer treatment
- PMID: 34367480
- PMCID: PMC8316873
- DOI: 10.4252/wjsc.v13.i7.841
Stem cell therapy: A paradigm shift in breast cancer treatment
Abstract
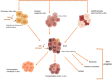
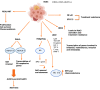
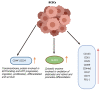
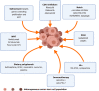
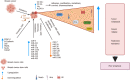
As per the latest Globocan statistics, the high prevalence rate of breast cancer in low- and middle-income countries has led to it becoming the most common cancer to be diagnosed, hence posing a major public health challenge. As per this data, more than 11.7% of the estimated new cancer cases in 2020 were due to breast cancer. A small but significant subpopulation of cells with self- renewing ability are present in the tumor stroma and have been given the nomenclature of cancer stem cells (CSCs). These cells display a high degree of plasticity owing to their ability to transition from the slowly cycling quiescent phase to the actively proliferating phenotype. This attribute of CSCs allows them to differentiate into various cell types having diverse functions. Breast CSCs have a pivotal role in development, metastasis, treatment resistance and relapse of breast cancers. This review focuses on the pathways regulating breast CSC maintenance and the current strategies that are being explored for directing the development of novel, targeted, therapeutic approaches for limiting and eradicating this aberrant stem cell population.
Keywords: Breast cancer stem cells; Inhibitors; Molecular genomics; Molecular pathways; Targeted therapy; Tumor microenvironment.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

