First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature
- PMID: 34367486
- PMCID: PMC8316863
- DOI: 10.4252/wjsc.v13.i7.944
First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature
Abstract
Background: Current clinical treatment options for symptomatic, partial-thickness rotator cuff tear (sPTRCT) offer only limited potential for true tissue healing and improvement of clinical results. In animal models, injections of adult stem cells isolated from adipose tissue into tendon injuries evidenced histological regeneration of tendon tissue. However, it is unclear whether such beneficial effects could also be observed in a human tendon treated with fresh, uncultured, autologous, adipose derived regenerative cells (UA-ADRCs). A specific challenge in this regard is that UA-ADRCs cannot be labeled and, thus, not unequivocally identified in the host tissue. Therefore, histological regeneration of injured human tendons after injection of UA-ADRCs must be assessed using comprehensive, immunohistochemical and microscopic analysis of biopsies taken from the treated tendon a few weeks after injection of UA-ADRCs.
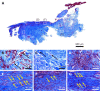
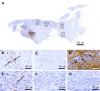
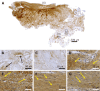
Case summary: A 66-year-old patient suffered from sPTRCT affecting the right supraspinatus and infraspinatus tendon, caused by a bicycle accident. On day 18 post injury [day 16 post magnetic resonance imaging (MRI) examination] approximately 100 g of abdominal adipose tissue was harvested by liposuction, from which approximately 75 × 106 UA-ADRCs were isolated within 2 h. Then, UA-ADRCs were injected (controlled by biplanar X-ray imaging) adjacent to the injured supraspinatus tendon immediately after isolation. Despite fast clinical recovery, a follow-up MRI examination 2.5 mo post treatment indicated the need for open revision of the injured infraspinatus tendon, which had not been treated with UA-ADRCs. During this operation, a biopsy was taken from the supraspinatus tendon at the position of the injury. A comprehensive, immunohistochemical and microscopic analysis of the biopsy (comprising 13 antibodies) was indicative of newly formed tendon tissue.
Conclusion: Injection of UA-ADRCs can result in regeneration of injured human tendons by formation of new tendon tissue.
Keywords: Adipose derived regenerative cells; Case report; Cell-based therapy at point of care; Partial-thickness rotator cuff tear; Stem cells; Tendon regeneration.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Alt EU is Executive Chair of InGeneron, Inc. (Houston, TX) and Chairman of the Board of Isar Klinikum (Munich, Germany). Alt C is Director of Science and Research of InGeneron GmbH (Munich, Germany) and of SciCoTec (Grünwald, Germany), the principal shareholder of InGeneron, Inc., which owns InGeneron GmbH (Munich, Germany). Schmitz C served as consultant to SciCoTec and the Alliance of Cardiovascular Researchers, and is Advisory Medical Director of InGeneron, Inc.
Figures












References
-
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–1767. - PubMed
-
- Ramírez J, Pomés I, Cabrera S, Pomés J, Sanmartí R, Cañete JD. Incidence of full-thickness rotator cuff tear after subacromial corticosteroid injection: a 12-week prospective study. Mod Rheumatol. 2014;24:667–670. - PubMed
-
- Hurley ET, Hannon CP, Pauzenberger L, Fat DL, Moran CJ, Mullett H. Nonoperative Treatment of Rotator Cuff Disease With Platelet-Rich Plasma: A Systematic Review of Randomized Controlled Trials. Arthroscopy. 2019;35:1584–1591. - PubMed
-
- Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, Saffarini M, Lädermann A. Efficacy of Platelet-Rich Plasma for the Treatment of Interstitial Supraspinatus Tears: A Double-Blinded, Randomized Controlled Trial. Am J Sports Med. 2019;47:1885–1892. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

