Increase in leaf organic acids to enhance adaptability of dominant plant species in karst habitats
- PMID: 34367574
- PMCID: PMC8328463
- DOI: 10.1002/ece3.7832
Increase in leaf organic acids to enhance adaptability of dominant plant species in karst habitats
Abstract
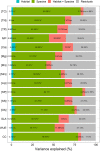
Estimation of leaf nutrient composition of dominant plant species from contrasting habitats (i.e., karst and nonkarst forests) provides an opportunity to understand how plants are adapted to karst habitats from the perspective of leaf traits. Here, we measured leaf traits-specific leaf area (SLA), concentrations of total carbon ([TC]), nitrogen ([TN]), phosphorus ([TP]), calcium ([Ca]), magnesium ([Mg]), manganese ([Mn]), minerals ([Min]), soluble sugars, soluble phenolics, lipids, and organic acids ([OA])-and calculated water-use efficiency (WUE), construction costs (CC), and N/P ratios, and searched for correlations between these traits of 18 abundant plant species in karst and nonkarst forests in southwestern China. Variation in leaf traits within and across the abundant species was both divergent and convergent. Leaf [TC], [Ca], [Min], [OA], and CC were habitat-dependent, while the others were not habitat- but species-specific. The correlations among [TN], [TP], SLA, [TC], CC, [Min], WUE, [OA], and CC were habitat-independent, and inherently associated with plant growth and carbon allocation; those between [CC] and [Lip], between [Ca] and [Mg], and between [Mg] and [WUE] were habitat-dependent. Habitat significantly affected leaf [Ca] and thus indirectly affected leaf [OA], [Min], and CC. Our results indicate that plants may regulate leaf [Ca] to moderate levels via adjusting leaf [OA] under both high and low soil Ca availability, and offer new insights into the abundance of common plant species in contrasting habitats.
Keywords: Guizhou; adaptation; calcium; mineral; nonkarst; nutrients.
© 2021 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ahrens, C. W. , Andrew, M. E. , Mazanec, R. A. , Ruthrof, K. X. , Challis, A. , Hardy, G. , Byrne, M. , Tissue, D. T. , & Rymer, P. D. (2020). Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecology and Evolution, 10(1), 232–248. 10.1002/ece3.5890 - DOI - PMC - PubMed
-
- Albert, C. H. , Grassein, F. , Schurr, F. M. , Vieilledent, G. , & Violle, C. (2011). When and how should intraspecific variability be considered in trait‐based plant ecology? Perspectives in Plant Ecology Evolution and Systematics, 13(3), 217–225. 10.1016/j.ppees.2011.04.003 - DOI
-
- Balachowski, J. A. , & Volaire, F. A. (2018). Implications of plant functional traits and drought survival strategies for ecological restoration. Journal of Applied Ecology, 55(2), 631–640. 10.1111/1365-2664.12979 - DOI
-
- Bjorkman, A. D. , Myers‐Smith, I. H. , Elmendorf, S. C. , Normand, S. , Rüger, N. , Beck, P. S. A. , Blach‐Overgaard, A. , Blok, D. , Cornelissen, J. H. C. , Forbes, B. C. , Georges, D. , Goetz, S. J. , Guay, K. C. , Henry, G. H. R. , HilleRisLambers, J. , Hollister, R. D. , Karger, D. N. , Kattge, J. , Manning, P. , … Weiher, E. (2018). Plant functional trait change across a warming tundra biome. Nature, 562(7725), 57–62. 10.1038/s41586-018-0563-7 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

