Abiraterone In Vitro Is Superior to Enzalutamide in Response to Ionizing Radiation
- PMID: 34367984
- PMCID: PMC8335570
- DOI: 10.3389/fonc.2021.700543
Abiraterone In Vitro Is Superior to Enzalutamide in Response to Ionizing Radiation
Abstract
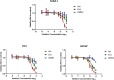
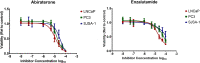
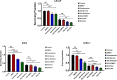
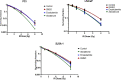
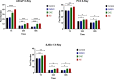
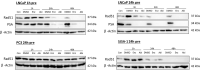
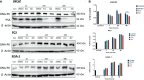
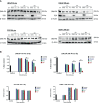
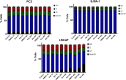
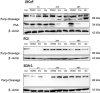
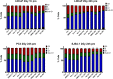
Abiraterone acetate and Enzalutamide are novel anti-androgens that are key treatments to improve both progression-free survival and overall survival in patients with metastatic castration-resistant prostate cancer. In this study, we aimed to determine whether combinations of AR inhibitors with radiation are additive or synergistic, and investigated the underlying mechanisms governing this. This study also aimed to compare and investigate a biological rationale for the selection of Abiraterone versus Enzalutamide in combination with radiotherapy as currently selection is based on consideration of side effect profiles and clinical experience. We report that AR suppression with Enzalutamide produces a synergistic effect only in AR-sensitive prostate models. In contrast, Abiraterone displays synergistic effects in combination with radiation regardless of AR status, alluding to potential alternative mechanisms of action. The underlying mechanisms governing this AR-based synergy are based on the reduction of key AR linked DNA repair pathways such as NHEJ and HR, with changes in HR potentially the result of changes in cell cycle distribution, with these reductions ultimately resulting in increased cell death. These changes were also shown to be conserved in combination with radiation, with AR suppression 24 hours before radiation leading to the most significant differences. Comparison between Abiraterone and Enzalutamide highlighted Abiraterone from a mechanistic standpoint as being superior to Abiraterone for all endpoints measured. Therefore, this provides a potential rationale for the selection of Abiraterone over Enzalutamide.
Keywords: DNA damage; abiraterone; androgen receptor; enzalutamide; prostate cancer; radiotherapy.
Copyright © 2021 Wright, Dunne, Alshehri, Redmond, Cole and Prise.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, et al. . Abiraterone Acetate Plus Prednisone Versus Placebo Plus Prednisone in Chemotherapy-Naive Men With Metastatic Castration-Resistant Prostate Cancer (COU-AA-302): Final Overall Survival Analysis of a Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Oncol (2015) 16(2):152–60. 10.1016/S1470-2045(14)71205-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

