Recent Advances in Mass Spectrometry-Based Glycomic and Glycoproteomic Studies of Pancreatic Diseases
- PMID: 34368082
- PMCID: PMC8342852
- DOI: 10.3389/fchem.2021.707387
Recent Advances in Mass Spectrometry-Based Glycomic and Glycoproteomic Studies of Pancreatic Diseases
Abstract
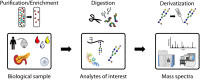
Modification of proteins by glycans plays a crucial role in mediating biological functions in both healthy and diseased states. Mass spectrometry (MS) has emerged as the most powerful tool for glycomic and glycoproteomic analyses advancing knowledge of many diseases. Such diseases include those of the pancreas which affect millions of people each year. In this review, recent advances in pancreatic disease research facilitated by MS-based glycomic and glycoproteomic studies will be examined with a focus on diabetes and pancreatic cancer. The last decade, and especially the last five years, has witnessed developments in both discovering new glycan or glycoprotein biomarkers and analyzing the links between glycans and disease pathology through MS-based studies. The strength of MS lies in the specificity and sensitivity of liquid chromatography-electrospray ionization MS for measuring a wide range of biomolecules from limited sample amounts from many sample types, greatly enhancing and accelerating the biomarker discovery process. Furthermore, imaging MS of glycans enabled by matrix-assisted laser desorption/ionization has proven useful in complementing histology and immunohistochemistry to monitor pancreatic disease progression. Advances in biological understanding and analytical techniques, as well as challenges and future directions for the field, will be discussed.
Keywords: diabetes; glycation; glycomics; glycoproteomics; glycosylation; mass spectrometry; pancreatic cancer; pancreatitis.
Copyright © 2021 Tabang, Ford and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Advances in mass spectrometry-based glycomics-An update covering the period 2017-2021.Electrophoresis. 2022 Jan;43(1-2):119-142. doi: 10.1002/elps.202100199. Epub 2021 Oct 27. Electrophoresis. 2022. PMID: 34505713 Review.
-
Liquid chromatography and capillary electrophoresis in glycomic and glycoproteomic analysis.Monatsh Chem. 2022;153(9):659-686. doi: 10.1007/s00706-022-02938-4. Epub 2022 Jun 21. Monatsh Chem. 2022. PMID: 35754790 Free PMC article. Review.
-
Advances in mass spectrometry-based glycomics.Electrophoresis. 2018 Dec;39(24):3063-3081. doi: 10.1002/elps.201800273. Epub 2018 Oct 9. Electrophoresis. 2018. PMID: 30199110 Review.
-
N-glycan MALDI Imaging Mass Spectrometry on Formalin-Fixed Paraffin-Embedded Tissue Enables the Delineation of Ovarian Cancer Tissues.Mol Cell Proteomics. 2016 Sep;15(9):3003-16. doi: 10.1074/mcp.M116.059816. Epub 2016 Jul 13. Mol Cell Proteomics. 2016. PMID: 27412689 Free PMC article.
Cited by
-
Characterization of Mesothelin Glycosylation in Pancreatic Cancer: Decreased Core Fucosylated Glycoforms in Pancreatic Cancer Patients' Sera.Biomedicines. 2022 Aug 10;10(8):1942. doi: 10.3390/biomedicines10081942. Biomedicines. 2022. PMID: 36009489 Free PMC article.
-
A Spin-Tip Enrichment Strategy for Simultaneous Analysis of N-Glycopeptides and Phosphopeptides from Human Pancreatic Tissues.J Vis Exp. 2022 May 4;(183):10.3791/63735. doi: 10.3791/63735. J Vis Exp. 2022. PMID: 35604151 Free PMC article.
-
Proteogenomic data and resources for pan-cancer analysis.Cancer Cell. 2023 Aug 14;41(8):1397-1406. doi: 10.1016/j.ccell.2023.06.009. Cancer Cell. 2023. PMID: 37582339 Free PMC article. Review.
-
Clinical glycoproteomics: methods and diseases.MedComm (2020). 2024 Oct 4;5(10):e760. doi: 10.1002/mco2.760. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39372389 Free PMC article. Review.
-
Tetrazine bioorthogonal chemistry derived in vivo imaging.Front Mol Biosci. 2022 Nov 16;9:1055823. doi: 10.3389/fmolb.2022.1055823. eCollection 2022. Front Mol Biosci. 2022. PMID: 36465558 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

