ARF GTPases and Their Ubiquitous Role in Intracellular Trafficking Beyond the Golgi
- PMID: 34368129
- PMCID: PMC8339471
- DOI: 10.3389/fcell.2021.679046
ARF GTPases and Their Ubiquitous Role in Intracellular Trafficking Beyond the Golgi
Abstract
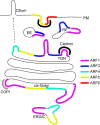
Molecular switches of the ADP-ribosylation factor (ARF) GTPase family coordinate intracellular trafficking at all sorting stations along the secretory pathway, from the ER-Golgi-intermediate compartment (ERGIC) to the plasma membrane (PM). Their GDP-GTP switch is essential to trigger numerous processes, including membrane deformation, cargo sorting and recruitment of downstream coat proteins and effectors, such as lipid modifying enzymes. While ARFs (in particular ARF1) had mainly been studied in the context of coat protein recruitment at the Golgi, COPI/clathrin-independent roles have emerged in the last decade. Here we review the roles of human ARF1-5 GTPases in cellular trafficking with a particular emphasis on their roles in post-Golgi secretory trafficking and in sorting in the endo-lysosomal system.
Keywords: ARF GTPase; COPI; Golgi; TGN; adaptors; clathrin; endosomes; membrane trafficking.
Copyright © 2021 Adarska, Wong-Dilworth and Bottanelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adolf F., Rhiel M., Hessling B., Gao Q., Hellwig A., Bethune J., et al. (2019). Proteomic Profiling of Mammalian COPII and COPI Vesicles. Cell Rep. 26 250–265e5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous