Meiotic Crossover Patterning
- PMID: 34368131
- PMCID: PMC8344875
- DOI: 10.3389/fcell.2021.681123
Meiotic Crossover Patterning
Abstract
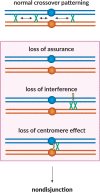
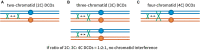
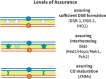
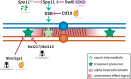
Proper number and placement of meiotic crossovers is vital to chromosome segregation, with failures in normal crossover distribution often resulting in aneuploidy and infertility. Meiotic crossovers are formed via homologous repair of programmed double-strand breaks (DSBs). Although DSBs occur throughout the genome, crossover placement is intricately patterned, as observed first in early genetic studies by Muller and Sturtevant. Three types of patterning events have been identified. Interference, first described by Sturtevant in 1915, is a phenomenon in which crossovers on the same chromosome do not occur near one another. Assurance, initially identified by Owen in 1949, describes the phenomenon in which a minimum of one crossover is formed per chromosome pair. Suppression, first observed by Beadle in 1932, dictates that crossovers do not occur in regions surrounding the centromere and telomeres. The mechanisms behind crossover patterning remain largely unknown, and key players appear to act at all scales, from the DNA level to inter-chromosome interactions. There is also considerable overlap between the known players that drive each patterning phenomenon. In this review we discuss the history of studies of crossover patterning, developments in methods used in the field, and our current understanding of the interplay between patterning phenomena.
Keywords: centromere; crossover assurance; interference; meiosis; recombination.
Copyright © 2021 Pazhayam, Turcotte and Sekelsky.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agostinho A., Meier B., Sonneville R., Jagut M., Woglar A., Blow J., et al. (2013). Combinatorial regulation of meiotic Holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 9:e1003591. 10.1371/journal.pgen.1003591 - DOI - PMC - PubMed
-
- Andersen S. L., Sekelsky J. (2010). Meiotic versus mitotic recombination: two different routes for double-strand break repair: the different functions of meiotic versus mitotic DSB repair are reflected in different pathway usage and different outcomes. Bioessays 32 1058–1066. 10.1002/bies.201000087 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

