Roles of MicroRNAs in Glucose and Lipid Metabolism in the Heart
- PMID: 34368265
- PMCID: PMC8339264
- DOI: 10.3389/fcvm.2021.716213
Roles of MicroRNAs in Glucose and Lipid Metabolism in the Heart
Abstract
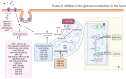
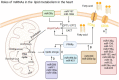
MicroRNAs (miRNAs) are small non-coding RNAs that participate in heart development and pathological processes mainly by silencing gene expression. Overwhelming evidence has suggested that miRNAs were involved in various cardiovascular pathological processes, including arrhythmias, ischemia-reperfusion injuries, dysregulation of angiogenesis, mitochondrial abnormalities, fibrosis, and maladaptive remodeling. Various miRNAs could regulate myocardial contractility, vascular proliferation, and mitochondrial function. Meanwhile, it was reported that miRNAs could manipulate nutrition metabolism, especially glucose and lipid metabolism, by regulating insulin signaling pathways, energy substrate transport/metabolism. Recently, increasing studies suggested that the abnormal glucose and lipid metabolism were closely associated with a broad spectrum of cardiovascular diseases (CVDs). Therefore, maintaining glucose and lipid metabolism homeostasis in the heart might be beneficial to CVD patients. In this review, we summarized the present knowledge of the functions of miRNAs in regulating cardiac glucose and lipid metabolism, as well as highlighted the miRNA-based therapies targeting cardiac glucose and lipid metabolism.
Keywords: glucose; heart; lipid; metabolism; microRNAs.
Copyright © 2021 Du, Zhao, Li, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
MicroRNAs regulate mitochondrial apoptotic pathway in myocardial ischemia-reperfusion-injury.Biomed Pharmacother. 2016 Dec;84:1635-1644. doi: 10.1016/j.biopha.2016.10.073. Epub 2016 Nov 6. Biomed Pharmacother. 2016. PMID: 27829552 Review.
-
MicroRNAs as Candidate Drug Targets for Cardiovascular Diseases.Curr Drug Targets. 2017;18(4):463-472. doi: 10.2174/1389450117666160301101221. Curr Drug Targets. 2017. PMID: 26926467 Review.
-
MicroRNA and Cardiovascular Diseases.Balkan Med J. 2020 Feb 28;37(2):60-71. doi: 10.4274/balkanmedj.galenos.2020.2020.1.94. Epub 2020 Feb 5. Balkan Med J. 2020. PMID: 32018347 Free PMC article.
-
Roles of microRNAs in carbohydrate and lipid metabolism disorders and their therapeutic potential.Biochimie. 2021 Aug;187:83-93. doi: 10.1016/j.biochi.2021.05.015. Epub 2021 Jun 1. Biochimie. 2021. PMID: 34082043 Review.
-
The Emerging Role of MitomiRs in the Pathophysiology of Human Disease.Adv Exp Med Biol. 2015;888:123-54. doi: 10.1007/978-3-319-22671-2_8. Adv Exp Med Biol. 2015. PMID: 26663182
Cited by
-
Model-informed experimental design recommendations for distinguishing intrinsic and acquired targeted therapeutic resistance in head and neck cancer.NPJ Syst Biol Appl. 2022 Sep 8;8(1):32. doi: 10.1038/s41540-022-00244-7. NPJ Syst Biol Appl. 2022. PMID: 36075912 Free PMC article.
-
MicroRNA and Heart Failure: A Novel Promising Diagnostic and Therapeutic Tool.J Clin Med. 2024 Dec 12;13(24):7560. doi: 10.3390/jcm13247560. J Clin Med. 2024. PMID: 39768484 Free PMC article. Review.
-
Cardiac Metabolism and MiRNA Interference.Int J Mol Sci. 2022 Dec 20;24(1):50. doi: 10.3390/ijms24010050. Int J Mol Sci. 2022. PMID: 36613495 Free PMC article. Review.
-
MicroRNA Profile, Putative Diagnostic Biomarkers and RNA-Based Therapies in the Inherited Lipid Storage Disease Niemann-Pick Type C.Biomedicines. 2023 Sep 23;11(10):2615. doi: 10.3390/biomedicines11102615. Biomedicines. 2023. PMID: 37892989 Free PMC article. Review.
-
Effects of astaxanthin on microRNA expression in a rat cardiomyocyte anoxia-reoxygenation model.Front Pharmacol. 2023 Feb 3;14:1103971. doi: 10.3389/fphar.2023.1103971. eCollection 2023. Front Pharmacol. 2023. PMID: 36817156 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

