Intercellular cGAMP transmission induces innate immune activation and tissue inflammation in Trex1 deficiency
- PMID: 34368651
- PMCID: PMC8326191
- DOI: 10.1016/j.isci.2021.102833
Intercellular cGAMP transmission induces innate immune activation and tissue inflammation in Trex1 deficiency
Abstract
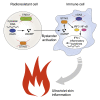
Intercellular transmission of the second messenger 2',3'-cGAMP, synthesized by the viral DNA sensor cGAMP synthase (cGAS), is a potent mode of bystander activation during host defense. However, whether this mechanism also contributes to cGAS-dependent autoimmunity remains unknown. Here, using a murine bone marrow transplantation strategy, we demonstrate that, in Trex1 -/- -associated autoimmunity, cGAMP shuttling from radioresistant to immune cells induces NF-κB activation, interferon regulatory factor 3 (IRF3) phosphorylation, and subsequent interferon signaling. cGAMP travel prevented myeloid cell and lymphocyte death, promoting their accumulation in secondary lymphoid tissue. Nonetheless, it did not stimulate B cell differentiation into autoantibody-producing plasmablasts or aberrant T cell priming. Although cGAMP-mediated bystander activation did not induce spontaneous organ disease, it did trigger interface dermatitis after UV light exposure, similar to cutaneous lupus erythematosus. These findings reveal that, in Trex1-deficiency, intercellular cGAMP transfer propagates cGAS signaling and, under conducive conditions, causes tissue inflammation.
Keywords: Cell biology; Immune response; Immunity; Immunology.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ablasser A., Hemmerling I., Schmid-Burgk J.L., Behrendt R., Roers A., Hornung V. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J. Immunol. 2014;192:5993–5997. - PubMed
-
- Aga E., Mukherjee A., Rane D., More V., Patil T., van Zandbergen G., Solbach W., Dandapat J., Tackenberg H., Ohms M. Type-1 interferons prolong the lifespan of neutrophils by interfering with members of the apoptotic cascade. Cytokine. 2018;112:21–26. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

