Restriction-free antimicrobial stewardship initiative targeting fluoroquinolone reduction across a regional health-system
- PMID: 34368680
- PMCID: PMC8335920
- DOI: 10.1016/j.infpip.2019.100019
Restriction-free antimicrobial stewardship initiative targeting fluoroquinolone reduction across a regional health-system
Abstract
Background: Fluoroquinolone (FQ) antibiotics have become a target of many antimicrobial stewardship programmes. Multiple post-marketing warnings from the Food and Drug Administration caution against use of this drug class for certain infections due to risk of harmful adverse effects outweighing benefit. Commonly employed strategies to affect antibiotic prescribing can be restrictive and without improvement in overall antibiotic appropriateness or decrease in collateral damage.
Aim: To develop a strategy for sustainable optimization of FQ antibiotics.
Setting: Multi-state health-system of 14 hospitals and medical centers.
Methods: The health-system antimicrobial stewardship program identified the opportunity to improve FQ utilization. In collaboration with our data and analytics team, specific targets of FQ use in pneumonia and chronic obstructive pulmonary disease were established. Face-to-face provider education and prospective audit and feedback were the mainstays of the campaign. Enhancements to the electronic medical record to support the initiative were also implemented.
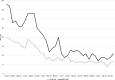
Findings: There was an overall decrease in FQ utilization by 56.9%. For pneumonia use of FQs decreased from 16.4% to 8.1% and in COPD changed from 29.6% to 9.7% over the same time period.
Conclusions: A non-restrictive FQ optimization initiative based on education and feedback decreased both FQ consumption and total antibiotic use across a large multi-hospital health-system.
Keywords: Antimicrobial stewardship; Fluoroquinolones; Health-system; Pharmacist.
© 2019 The Authors.
Figures


References
-
- Dellit T.H., Owens R.C., Mcgowan J.E., Gerding D.N., Weinstein R.A., Burke J.P. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. - PubMed
-
- Food and Drug Administration (FDA) 2016. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects.https://www.fda.gov/Drugs/DrugSafety/ucm511530 Published.
-
- Food and Drug Administration (FDA) 2018. FDA Drug Safety Communication: FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes.https://www.fda.gov/downloads/Drugs/DrugSafety/UCM612834.pdf Published.
-
- Food and Drug Administration (FDA) 2018. FDA Drug Safety Communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients.https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-i... Published.
-
- Pitiriga V., Vrioni G., Saroglou G., Tsakris A. The impact of antibiotic stewardship programs in combating quinolone resistance: a systematic review and recommendations for more efficient interventions. Adv Ther. 2017;34:854–865. - PubMed
LinkOut - more resources
Full Text Sources

