Automated PET-RAFT Polymerization Towards Pharmaceutical Amorphous Solid Dispersion Development
- PMID: 34368765
- PMCID: PMC8336633
- DOI: 10.1021/acsapm.0c01376
Automated PET-RAFT Polymerization Towards Pharmaceutical Amorphous Solid Dispersion Development
Abstract
In pharmaceutical oral drug delivery development, about 90% of drugs in the pipeline have poor aqueous solubility leading to severe challenges with oral bioavailability and translation to effective and safe drug products. Amorphous solid dispersions (ASDs) have been utilized to enhance the oral bioavailability of poorly soluble active pharmaceutical ingredients (APIs). However, a limited selection of regulatory-approved polymer excipients exists for the development and further understanding of tailor-made ASDs. Thus, a significant need exists to better understand how polymers can be designed to interact with specific API moieties. Here, we demonstrate how an automated combinatorial library approach can be applied to the synthesis and screening of polymer excipients for the model drug probucol. We synthesized a library of 25 random heteropolymers containing one hydrophilic monomer (2-hydroxypropyl acrylate (HPA)) and four hydrophobic monomers at varied incorporation. The performance of ASDs made by a rapid film casting method was evaluated by dissolution using ultra-performance liquid chromatography (UPLC) sampling at various time points. This combinatorial library and rapid screening strategy enabled us to identify a relationship between polymer hydrophobicity, monomer hydrophobic side group geometry, and API dissolution performance. Remarkably, the most effective synthesized polymers displayed slower drug release kinetics compared to industry standard polymer excipients, showing the ability to modulate the drug release profile. Future coupling of high throughput polymer synthesis, high throughput screening (HTS), and quantitative modeling would enable specification of designer polymer excipients for specific API functionalities.
Keywords: amorphous solid dispersions; combinatorial polymer synthesis; drug precipitation inhibition; oral drug delivery; polymer excipients.
Figures


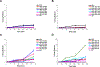
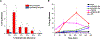



References
-
- Ting JM; Porter III WW; Mecca JM; Bates FS; Reineke TM, Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery. Bioconjugate Chem 2018, 29, 939–952. - PubMed
-
- Loftsson T; Brewster ME, Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol 2010, 62, 1607–1621. - PubMed
-
- Thompson LA; Ellman JA, Synthesis and Applications of Small Molecule Libraries. Chem. Rev 1996, 96, 555–600. - PubMed
-
- Gordon EM; Gallop MA; Patel DV, Strategy and Tactics in Combinatorial Organic synthesis. Applications to Drug Discovery. Accounts Chem. Res 1996, 29, 144–154.
Grants and funding
LinkOut - more resources
Full Text Sources

