HO‑1 knockdown upregulates the expression of VCAM‑1 to induce neutrophil recruitment during renal ischemia‑reperfusion injury
- PMID: 34368855
- PMCID: PMC8416149
- DOI: 10.3892/ijmm.2021.5018
HO‑1 knockdown upregulates the expression of VCAM‑1 to induce neutrophil recruitment during renal ischemia‑reperfusion injury
Abstract
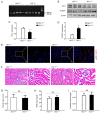
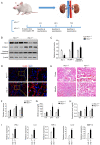
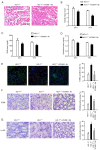
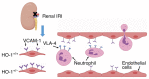
Heme oxygenase‑1 (HO‑1) has been reported to be upregulated following renal ischemia‑reperfusion injury (IRI) and plays a key cytoprotective role; however, the underlying molecular mechanisms of its protective effects remain poorly understood. In the present study, in order to further elucidate the molecular mechanisms underlying the cytoprotective role of HO‑1 in renal IRI, HO‑1+/+ and HO‑1+/‑ mice were subjected to renal ischemia and subsequent reperfusion followed by the analysis of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, the severity of histological changes, HO‑1 and vascular cell adhesion molecule‑1 (VCAM‑1) protein expression, the mRNA expression of inflammatory factors and the effects of VCAM‑1 blockade. The results of the present study demonstrated that the upregulated expression levels of VCAM‑1 in HO‑1+/‑ mice during IRI increased the extent of renal tissue damage and activated the inflammatory response. These effects were subsequently reversed following infusion with an anti‑VCAM‑1 antibody. In addition, the upregulated expression of VCAM‑1 in mouse glomerulus vascular endothelial cells isolated from HO‑1+/‑ mice increased the adhesion and migration of neutrophils, effects which were also reversed upon incubation with an anti‑VCAM‑1 antibody. These results indicated that HO‑1 knockdown may upregulate the expression of VCAM‑1 during renal IRI, resulting in increased neutrophil recruitment and the activation of the inflammatory response, thereby exacerbating renal IRI. The present study thus highlights the regulatory mechanisms of HO‑1 in renal IRI and provides a potential target for the clinical treatment of IRI following renal transplantation.
Keywords: adhesion; heme oxygenase‑1; migration; neutrophil recruitment; renal ischemia‑reperfusion injury; vascular cell adhesion molecule‑1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Sheashaa H, Lotfy A, Elhusseini F, Aziz AA, Baiomy A, Awad S, Alsayed A, El-Gilany AH, Saad MA, Mahmoud K, et al. Protective effect of adipose-derived mesenchymal stem cells against acute kidney injury induced by ischemia-reperfusion in Sprague-Dawley rats. Exp Ther Med. 2016;11:1573–1580. doi: 10.3892/etm.2016.3109. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

