Oxymatrine attenuates oxidized low‑density lipoprotein‑induced HUVEC injury by inhibiting NLRP3 inflammasome‑mediated pyroptosis via the activation of the SIRT1/Nrf2 signaling pathway
- PMID: 34368883
- PMCID: PMC8416146
- DOI: 10.3892/ijmm.2021.5020
Oxymatrine attenuates oxidized low‑density lipoprotein‑induced HUVEC injury by inhibiting NLRP3 inflammasome‑mediated pyroptosis via the activation of the SIRT1/Nrf2 signaling pathway
Abstract
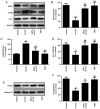
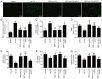
Oxymatrine, a quinolizidine alkaloid isolated from the traditional Chinese herb Sophora flavescens Aiton, has been demonstrated to exert anti‑inflammatory and atherosclerotic effects, but the molecular mechanism has yet to be elucidated. Accumulating evidence indicates an important role of NLR family pyrin domain containing 3 (NLRP3) inflammasome‑mediated pyroptosis in the pathogenesis of atherosclerosis. The present study was undertaken to investigate whether oxymatrine attenuates oxidized low‑density lipoprotein (ox‑LDL)‑induced human umbilical vein endothelial cell (HUVEC) injury, an in vitro cell model of atherosclerosis, by inhibiting NLRP3 inflammasome‑mediated pyroptosis, and elucidate the role of the sirtuin (SIRT)1/nuclear factor‑erythroid 2‑related factor 2 (Nrf2) signaling pathway in this process. Cell viability and cytotoxicity were detected by CCK‑8 assay and a lactate dehydrogenase (LDH) assay kit. Cell apoptosis was detected by flow cytometry. Reactive oxygen species (ROS) generation was detected using a ROS assay kit. The malondialdehyde (MDA) content, mitochondrial membrane potential (MMP) level, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH‑Px) activities were determined using commercial kits. The inflammatory cytokines levels were measured by ELISA and protein expression was monitored by western blot analysis. The results revealed that oxymatrine alleviated ox‑LDL‑induced cytotoxicity and apoptosis. Concurrently, oxymatrine inhibited ox‑LDL‑induced NLRP3 inflammasome‑mediated pyroptosis in HUVECs, as evidenced by the significant decreases in the expression of NLRP3, apoptosis‑associated speck‑like protein containing a C‑terminal caspase recruitment domain (ASC), cleaved caspase‑1, interleukin (IL)‑1β and IL‑18 in HUVECs. In addition, NLRP3 siRNA transfection efficiently suppressed ox‑LDL‑induced pyroptosis and HUVEC injury. Furthermore, oxymatrine promoted SIRT1/Nrf2 signaling pathway activation in HUVECs subjected to ox‑LDL treatment, and SIRT1 deficiency induced by SIRT1 siRNA transfection abolished the protective effect of oxymatrine against ox‑LDL‑induced injury. SIRT1 siRNA also mitigated the oxymatrine‑induced decreases in ROS generation and MDA content, and the increases in MMP as well as the activities of SOD, CAT and GSH‑Px in HUVECs. Moreover, SIRT1 siRNA transfection blocked the inhibitory effect of oxymatrine on NLRP3 inflammasome‑mediated pyroptosis in ox‑LDL‑treated HUVECs. Collectively, these results indicated that oxymatrine may attenuate ox‑LDL‑induced HUVEC injury by inhibiting NLRP3 inflammasome‑mediated pyroptosis via activating the SIRT1/Nrf2 signaling pathway.
Keywords: NLRP3 inflammasome‑mediated pyroptosis; atherosclerosis; endothelial cell injury; oxymatrine; sirtuin 1/nuclear factor‑erythroid 2‑related factor 2 signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sørensen K, Skjelland M, Espevik T, Aukrust P, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5:e003031. doi: 10.1161/JAHA.115.003031. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

