How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment
- PMID: 34368939
- PMCID: PMC8516790
- DOI: 10.1007/s40273-021-01065-y
How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment
Abstract
Background: Debate over the viability of the current commercial research and development (R&D) model is ongoing. A controversial theme is the cost of bringing a new molecular entity (NME) to market.
Objective: Our aim was to evaluate the range and suitability of published R&D cost estimates as to the degree to which they represent the actual costs of industry.
Methods: We provided a systematic literature review based on articles found in the Pubmed, Embase and EconLit electronic databases, and in a previously published review. Articles published before March 2020 that estimated the total R&D costs were included (22 articles with 45 unique cost estimates). We included only literature in which the methods used to collect the information and to estimate the R&D costs were clearly described; therefore, three reports were excluded. We extracted average pre-launch R&D costs per NME and converted the values to 2019 US dollars (US$) using the gross domestic product (GDP) price deflator. We appraised the suitability of the R&D estimated costs by using a scoring system that captures three domains: (1) how success rates and development time used for cost estimation were obtained; (2) whether the study considered potential sources contributing to the variation in R&D costs; and (3) what the components of the cost estimation were.
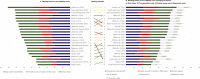
Results: Estimates of total average capitalized pre-launch R&D costs varied widely, ranging from $161 million to $4.54 billion (2019 US$). Therapeutic area-specific estimates were highest for anticancer drugs (between $944 million and $4.54 billion). Our analysis identified a trend of increasing R&D costs per NME over time but did not reveal a relation between cost estimates and study ranking when the suitability scores were assessed. We found no evidence of an increase in suitability scores over time.
Conclusion: There is no universally correct answer regarding how much it costs, on average, to research and develop an NME. Future studies should explicitly address previously neglected variables, which likely explain some variability in estimates, and consider the trade-off between the transparency and public accessibility of data and their specificity. Use of our proposed suitability scoring system may assist in addressing such issues.
© 2021. The Author(s).
Conflict of interest statement
Michael Schlander, Karla Hernandez-Villafuerte, Chih-Yuan Cheng, Jorge Mestre-Ferrandiz, and Michael Baumann have no conflicts of interest to report.
Figures



References
-
- Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10(6):428. - PubMed
-
- Barker R. A flexible blueprint for the future of drug development. Lancet. 2010;375(9712):357–359. - PubMed
-
- Gambardella A, Orsenigo L, Pammolli F, Lacetera N, Magazzini L, Mariani M, et al. Global competitiveness in pharmaceuticals: a European perspective. Brussels: European Commission; 2000.
-
- Backfisch M. Have pharmaceutical R&D project success rates decreased? A critical review and new empirical results. In Joint discussion paper series in economics. 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

