Chronic non-invasive ventilation for chronic obstructive pulmonary disease
- PMID: 34368950
- PMCID: PMC8407093
- DOI: 10.1002/14651858.CD002878.pub3
Chronic non-invasive ventilation for chronic obstructive pulmonary disease
Abstract
Background: Chronic non-invasive ventilation (NIV) is increasingly being used to treat people with COPD who have respiratory failure, but the evidence supporting this treatment has been conflicting.
Objectives: To assess the effects of chronic non-invasive ventilation at home via a facial mask in people with COPD, using a pooled analysis of IPD and meta-analysis.
Search methods: We searched the Cochrane Airways Register of Trials, MEDLINE, Embase, PsycINFO, CINAHL, AMED, proceedings of respiratory conferences, clinical trial registries and bibliographies of relevant studies. We conducted the latest search on 21 December 2020.
Selection criteria: We included randomised controlled trials (RCTs) comparing chronic NIV for at least five hours per night for three consecutive weeks or more (in addition to standard care) versus standard care alone, in people with COPD. Studies investigating people initiated on NIV in a stable phase and studies investigating NIV commenced after a severe COPD exacerbation were eligible, but we reported and analysed them separately. The primary outcomes were arterial blood gases, health-related quality of life (HRQL), exercise capacity (stable COPD) and admission-free survival (post-exacerbation COPD). Secondary outcomes for both populations were: lung function, COPD exacerbations and admissions, and all-cause mortality. For stable COPD, we also reported respiratory muscle strength, dyspnoea and sleep efficiency.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. After inclusion of a study, we requested the IPD. We analysed continuous and time-to-event data using linear- and cox-regression mixed-effect models with a random effect on study level. We analysed dichotomous IPD using generalised estimating equations. We adjusted all models for age and sex. We assessed changes in outcomes after three and 12 months. We also conducted a meta-analysis on aggregated trial data.
Main results: We included 14 new RCTs in this review update, in addition to the seven previously included. Seventeen studies investigated chronic NIV in stable COPD and four studies investigated chronic NIV commenced after a severe COPD exacerbation. Three studies compared NIV to sham continuous positive airway pressure (2 to 4 cmH2O). Seven studies used a nasal mask, one study used an oronasal mask and eight studies used both interfaces. Five studies did not report the interface. The majority of trials (20/21) were at high risk of performance bias due to an unblinded design. We considered 11 studies to have a low risk of selection bias and 13 to have a low risk of attrition bias. We collected and analysed the IPD from 13 stable COPD studies (n = 778, 68% of the participants included) and from three post-exacerbation studies (n = 364, 96% of the participants included). In the stable COPD group, NIV probably results in a minor benefit on the arterial partial pressure of oxygen (PaO2) after three months (adjusted mean difference (AMD) 0.27 kPa, 95% CI 0.04 to 0.49; 9 studies, 271 participants; moderate-certainty evidence), but there was little to no benefit at 12 months (AMD 0.09 kPa, 95% CI -0.23 to 0.42; 3 studies, 171 participants; low-certainty evidence). The arterial partial pressure of carbon dioxide (PaCO2) was reduced in participants allocated to NIV after three months (AMD -0.61 kPa, 95% CI -0.77 to -0.45; 11 studies, 475 participants; high-certainty evidence) and persisted up to 12 months (AMD -0.42 kPa, 95% CI -0.68 to -0.16; 4 studies, 232 participants; high-certainty evidence). Exercise capacity was measured with the 6-minute walking distance (minimal clinical important difference: 26 m). There was no clinically relevant effect of NIV on exercise capacity (3 months: AMD 15.5 m, 95% CI -0.8 to 31.7; 8 studies, 330 participants; low-certainty evidence; 12 months: AMD 26.4 m, 95% CI -7.6 to 60.5; 3 studies, 134 participants; very low-certainty evidence). HRQL was measured with the Severe Respiratory Insufficiency and the St. Georges's Respiratory Questionnaire and may be improved by NIV, but only after three months (3 months: standardised mean difference (SMD) 0.39, 95% CI 0.15 to 0.62; 5 studies, 259 participants; very low-certainty evidence; 12 months: SMD 0.15, 95% CI -0.13 to 0.43; 4 studies, 200 participants; very low-certainty evidence). Lastly, the risk for all-cause mortality is likely reduced by NIV (adjusted hazard ratio (AHR) 0.75, 95% CI 0.58 to 0.97; 3 studies, 405 participants; moderate-certainty evidence). In the post-exacerbation COPD group, there was little to no benefit on the PaO2 after three months, but there may be a slight decrease after 12 months (3 months: AMD -0.10 kPa, 95% CI -0.65 to 0.45; 3 studies, 234 participants; low-certainty evidence; 12 months: -0.27 kPa, 95% CI -0.86 to 0.32, 3 studies; 170 participants; low-certainty evidence). The PaCO2 was reduced by NIV at both three months (AMD -0.40 kPa, 95% CI -0.70 to -0.09; 3 studies, 241 participants; moderate-certainty evidence) and 12 months (AMD -0.52 kPa, 95% CI -0.87 to -0.18; 3 studies, 175 participants; high-certainty evidence). NIV may have little to no benefit on HRQL (3 months: SMD 0.25, 95% CI -0.01 to 0.51; 2 studies, 219 participants; very low-certainty evidence; 12 months: SMD 0.25, 95% -0.06 to 0.55; 2 studies, 164 participants; very low-certainty evidence). Admission-free survival seems improved with NIV (AHR 0.71, 95% CI 0.54 to 0.94; 2 studies, 317 participants; low-certainty evidence), but the risk for all-cause mortality does not seem to improve (AHR 0.97, 95% CI 0.74 to 1.28; 2 studies, 317 participants; low-certainty evidence).
Authors' conclusions: Regardless of the timing of initiation, chronic NIV improves daytime hypercapnia. In addition, in stable COPD, survival seems to be improved and there might be a short term HRQL benefit. In people with persistent hypercapnia after a COPD exacerbation, chronic NIV might prolong admission-free survival without a beneficial effect on HRQL. In stable COPD, future RCTs comparing NIV to a control group receiving standard care might no longer be warranted, but research should focus on identifying participant characteristics that would define treatment success. Furthermore, the optimal timing for initiation of NIV after a severe COPD exacerbation is still unknown.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
T Raveling: none known.
J Vonk: none known.
FM Struik: none known.
R Goldstein: none known.
HAM Kerstjens: none known.
PJ Wijkstra: none known.
ML Duiverman: none known.
Figures
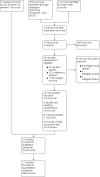
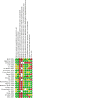
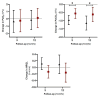
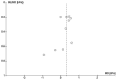
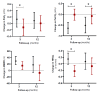



























Update of
-
Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Jun 13;2013(6):CD002878. doi: 10.1002/14651858.CD002878.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2021 Aug 9;8:CD002878. doi: 10.1002/14651858.CD002878.pub3. PMID: 23766138 Free PMC article. Updated.
References
References to studies included in this review
Bhatt 2013 {published and unpublished data}
-
- NCT01722773. Trial of non-invasive ventilation for stable COPD. clinicaltrials.gov/ct2/show/NCT01722773 (first received 7 November 2012).
Casanova 2000 {published and unpublished data}
-
- Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with COPD. Chest 2000;118(6):1582-90. - PubMed
-
- Casanova C, Tost L, Soriano E, Abreu J, Hernandez MC, Velasco MV, et al. Night time nasal ventilation with BiPAPs for severe COPD in stable phase. Archivos de bronconeumologia 1997;33((suppl 1)):44-46.
-
- Macario C, Casanova L, Tost E, Soriano E, Abreu J, Velasco MV, et al. Nocturnal nasal ventilation with BiPAPs in severe stable COPD. European Respiratory Journal 1997;10 Suppl 25(Suppl 25):78S.
-
- Macario CC, Abreu J, Soriano E, Tost L, Acosta O, Garcia Talavera I, et al. Nocturnal nasal ventilation with BiPAP in stable severe COPD: a long term study. European Respiratory Journal 1995;8 Suppl 19(Suppl 19):402S.
Cheung 2010 {published and unpublished data}
-
- Cheung AP, Chan VL, Liong JT, Lam JY, Leung WS, Lin A, et al. A pilot trial of non-invasive home ventilation after acidotic respiratory failure in chronic obstructive pulmonary disease. International Journal of Tuberculosis and Lung Disease 2010;14(5):642-9. [PMID: ] - PubMed
-
- Cheung AP, Chan VL, Liong JT, Lam JY, Leung WS, Lin A, et al. A randomised controlled trial of continuation of home non-invasive ventilation vs sham ventilation in survivors of acute hypercapnic respiratory failure in chronic obstructive airway disease. In: Respirology. Vol. 13. 2008:A120 (012-04).
-
- Chu C-M, Cheung A, Chan V, Liong J, Leung W-S, Lin A. A randomised trial of home non-invasive ventilation vs sham ventilation in survivors of acute hypercapnic respiratory failure in COPD. In: European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin. 2008:3665.
-
- Chu CM, Cheung AP, Chan VL, Leung WS, Lin AW. A randomized trial of home non-invasive ventilation vs sham ventilation in survivors of acute hypercapnic respiratory failure in COPD. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008:A767.
Clini 2002 {published and unpublished data}
-
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. European Respiratory Journal 2002;3:529-38. - PubMed
-
- Clini E, Sturani C. A multicentric long-term study of noninvasive pressure support ventilation (NSPV) in hypercapnic COPD patients. In: European Respiratory Journal. Vol. 12. 1998:297S.
Cui 2019 {published data only}
-
- Limin C, Haixia L, Lei S. Multidisciplinary respiratory rehabilitation in combination with non-invasive positive pressure ventilation in the treatment of elderly patients with severe chronic obstructive pulmonary disease.. Pakistan Journal of Medical Science 2019;35(2):500-5. [DOI: 10.12669/pjms.35.2.459] - DOI - PMC - PubMed
De Backer 2011 {published data only}
-
- De Backer L, De Backer WA, Vos W, De Backer J, Daems D, Claes R, et al. Factors Determining Physiological Effects Of Non-Invasive Ventilation In Hypercapnic COPD Patients. In: European Respiratory Society 21st Annual Congress; 2011 Sep 24-28; Amsterdam. Vol. 183. 2011:A4575.
-
- De Backer L, Vos W, Dieriks B, Daems D, Verhulst S, Vinchurkar S, et al. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot study. International Journal of Chronic Obstructive Pulmonary Disease 2011;6:615-24. [DOI: 10.2147/COPD.S22823] - DOI - PMC - PubMed
Duiverman 2008 {published and unpublished data}
-
- Duiverman ML, Bladder G, Wempe JB, Kerstjens HA, Zijlstra JG, Wijkstra PJ. Chronic ventilatory support improves the outcomes of rehabilitation in hypercapnic COPD patients. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008.
-
- Duiverman ML, Wempe JB, Bladder G, Vonk J, Kerstjens HA, Wijkstra PJ. Two-year nocturnal noninvasive ventilation added to rehabilitation in hypercapnic COPD patients. American Journal of Respiratory and Critical Care Medicine 2010;B44(181):A3056. [DOI: ]
-
- Duiverman ML, Wempe JB, Bladder G, Jansen DF, Kerstjens HAM, Zijlstra JG, et al. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 2008;63(12):1052-7. [PMID: ] - PubMed
Eman Shebl 2015 {published data only}
-
- Eman Shebl R, Abderaboh MM. Bi-level positive airway pressure ventilation for patients with stable hypercapnic chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases and Tuberculosis 2015;64(2):395-8. [DOI: ]
Garrod 2000 {published data only}
-
- Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;162:1335-41. - PubMed
-
- Garrod R, Mikelsous C, Paul EA, Wedzicha JA. Randomised controlled trial of domiciliary non-invasive positive pressure ventilation and physical training in severe COPD. American Journal of Respiratory and Critical Care Medicine 2000;61(Suppl 3):A255. - PubMed
Gay 1996 {published data only}
-
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a three-month controlled trial. Mayo Clinic Proceedings 1996;71(6):533-42. - PubMed
Köhnlein 2014 {published and unpublished data}
-
- Kohnlein T, Criee CP, Kohler D, Welte T, Laier-Groeneveld G. Multicenter Study on “Non-Invasive Ventilation in Patients with Severe Chronic Obstructive Pulmonary Disease and Emphysema(COPD)” [Multizentrische Studie: „Nicht-invasive Beatmung bei Patienten mit schwerer chronisch obstruktiver Bronchitis und Emphysem (COPD)”]. Pneumologie 2004;58(8):566-9. [DOI: 10.1055/s-2004-818542] - DOI - PubMed
-
- Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respiratory Medicine 2014;2(9):698-705. [PMID: ] - PubMed
Ma 2019 {published data only}
-
- Ma T, Hu Y, Yan B. The analysis of non-invasive positive pressure ventilation in treating COPD patients accompanied with chronic respiratory failure. American Journal of Respiratory and Critical Care Medicine 2019;199(B44):A3344. [DOI: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A3344] - DOI
Martin‐Marquez 2014 {published and unpublished data}
-
- Marquez-Martin E, Cejudo P, Lopez-Campos JL, Rodriguez A, Valencia B, Barrot E, et al. Home mechanical ventilation and respiratory rehabilitation: Influence in respiratory functional test and exercise capacity. European Respiratory Journal 2012;40(Suppl 56):294s [P1710].
-
- Marquez-Martin E, Ruiz FO, Ramos PC, Lopez-Campos JL, Azcona BV, Cortes EB. Randomized trial of non-invasive ventilation combined with exercise training in patients with chronic hypercapnic failure due to chronic obstructive pulmonary disease. Respiratory Medicine 2014;108(12):1741-51. - PubMed
McEvoy 2009 {published data only}
-
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009;64(7):561-6. - PubMed
Meecham Jones 1995 {published data only}
-
- Meecham Jones D, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. American Journal of Respiratory and Critical Care Medicine 1995;152(152):538-44. - PubMed
-
- Meecham Jones DJ, Paul EA, Wedzicha JA. Nocturnal nasal pressure support ventilation with supplemental oxygen therapy compared with oxygen therapy alone in stable hypercapnic COPD — a randomised controlled study: effects on sleep and overnight blood gases. European Respiratory Journal 1994;7(Suppl 18):461s.
Murphy 2017 {published and unpublished data}
-
- Murphy P, Arbane G, Bourke S, Calverley P, Dowson L, Duffy N, et al. Improving admission free survival with home mechanical ventilation (HMV) and home oxygen therapy (HOT) following life threatening COPD exacerbations: hoT-HMV UK Trial NCT00990132. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
-
- Murphy PB, Arbane G, Bisquera A, Hart N. Home mechanical ventilation (HMV) and home oxygen therapy (HOT) following an acute exacerbation of COPD in patients with persistent hypercapnia: predicting 1 year admission-free survival in the hot-HMV UK trial. In: British Thoracic Society Winter Meeting; 2017 Dec 6–8; London. Vol. Supplement 3. 2017:A26-7.
-
- Murphy PB, Arbane G, Bourke S, Calverley P, Crooks A, Dowson L, et al. Hot-HMV UK trial secondary outcome analysis: early readmission is reduced by the addition of home mechanical ventilation to home oxygen therapy in COPD patients with chronic respiratory failure following a life-threatening exacerbation. Thorax 2016;71:A68-9. [DOI: 10.1136/thoraxjnl-2016-209333.121] - DOI
-
- Murphy PB, Arbane G, Patout M, Hart N. Results from UK HOT-HMV Trial: clinical importance of recovery of hypercapnia following acute life-threatening exacerbation of COPD. European Respiratory Journal 2018;52((suppl 62)):PA1679. [DOI: 10.1183/13993003.congress-2018.PA1679] - DOI
-
- Murphy PB, Arbane G, Phillips R, Hart N. Home mechanical ventilation (HMV) and home oxygen therapy (HOT) following an acute exacerbation of COPD in patients with persistent hypercapnia: results of the per protocol analysis from the hot-HMV UK trial. In: British Thoracic Society Winter Meeting; 2017 Dec 6–8; London. Vol. Supplement 3. 2017:A25-A6.
Schneeberger 2017 {published data only}
-
- Schneeberger T, Stegemann A, Schoenheit-Kenn U, Jarosch I, Gloeckl R, Kohnlein T, et al. Nocturnal non invasive ventilation as an adjunct for pulmonary rehabilitation in patients with very severe COPD — a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2017;195:A2851.
Sin 2007 {published data only}
-
- Sin DD, Wong E, Mayers I, Lien DC, Feeny D, Cheung H, et al. Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD. Chest 2007;131(1):156-63. - PubMed
Struik 2014 {published and unpublished data}
-
- Struik FM, Kerstjens HA, Bladder G, Sprooten R, Zijnen M, Asin J, et al. Nocturnal noninvasive ventilation in COPD patients who remain hypercapnic after ventilatory support for an acute respiratory failure: a randomized, controlled, parallel-group study. In: American Journal of Respiratory and Critical Care Medicine. Vol. 189. 2014:A1174. - PubMed
-
- Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Sin JA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014;69(9):826-34. - PubMed
Strumpf 1991 {published data only}
-
- Strumpf DA, Millman RP, Carlisle CC. Nocturnal positive pressure ventilation via nasal mask in patients with COPD. American Review of Respiratory Disease 1991;144:1234-9. - PubMed
Zhou 2017 {published data only}
-
- NCT02499718. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02499718 (first received 16 July 2015).
-
- Zhou L, Li X, Guan L, Chen J, Guo B, Wu W, et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:1279-86. - PMC - PubMed
References to studies excluded from this review
Ali 2012 {unpublished data only}
-
- Ali M S, Talwar D, Singh M. Nocturnal noninvasive ventilation improves muscle strength in stable COPD patients with respiratory failure DBP - EM:201332. Thorax 2013;67(Suppl 2):A165 (P229).
Chiang 2004 {published data only}
-
- Chiang LL, Liu CY, Ho SC, Sheng TF, Yu CT, Lin HC, et al. Efficacy of nocturnal nasal positive pressure ventilation in hypercapnic patients with severe obstructive lung diseases. Chang Gung Medical Journal 2004;27(2):98-106. - PubMed
-
- Chiang LL, Yu CT, Liu CY, Lo YL, Kuo HP, Lin HC. Six-month nocturnal nasal positive pressure ventilation improves respiratory muscle capacity and exercise endurance in patients with chronic hypercapnic respiratory failure. Journal of the Formosan Medical Association/Taiwan 2006;105(6):459-67. - PubMed
Clini 1998 {published data only}
-
- Clini E, Strurani C, Porta R. Outcome of COPD patients performing nocturnal non-invasive mechanical ventilation. Respiratory Medicine 1998;92:1215-22. - PubMed
Diaz 1999 {published data only}
-
- Diaz O, Gallardo J, Ramos J, Torrealba B, Lisboa C. Non-invasive mechanical ventilation in severe stable hypercapnic COPD patients: a prospective randomised clinical trial. American Journal of Respiratory and Critical Care Medicine 1999;159:A295.
Funk 2011 {published data only}
Kamei 1999 {published data only}
-
- Kamei M. Effectiveness of noninvasive positive-pressure ventilation for patients with chronic stable hypercapnic respiratory failure. Nihon Koyuki Gakkai Zasshi 1999;37(11):886-92. - PubMed
Lin 1996 {published data only}
-
- Lin CC. Comparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPD. American Journal of Respiratory and Critical Care Medicine 1996;154:353-8. - PubMed
-
- Lin CC. Effect of nocturnal nasal positive pressure ventilation plus oxygen combined therapy and oxygen monotherapy in severe COPD patients. European Respiratory Journal 1995;8(Suppl 19):210S.
Nicolini 2014 {published data only}
-
- Nicolini A, Mollar E, Grecchi B, Landucci N. Comparison of Intermittent positive pressure breathing and temporary positive expiratory pressure in patients with severe chronic obstructive pulmonary disease. Archivos de bronconeumologia 2017;50:18-24. [DOI: 10.1016/j.arbres.2013.07.019] - DOI - PubMed
References to studies awaiting assessment
Baturova 2013 {published data only}
-
- Baturova V, Zolotova E, Svet A, Nesterov A, K Dalgatova. Effectiveness of non-invasive ventilation in stable chronic obstructive pulmonary disease with nocturnal desaturation: Prospective open-label randomized parallel-group study. In: European Respiratory Society 23rd Annual Congress; 2013 Sep 7-11; Barcelona. 2013.
Guan 2018 {published data only}
-
- Guan L, Zhou L, Chen R. Home noninvasive ventilation with built-in software in chronic hypercapnic COPD patients: a mid-term prospective, multicenter, randomized, controlled trial. European Respiratory Journal 2018;52((suppl 62)):PA1677. [DOI: 10.1183/13993003.congress-2018.PA1677] - DOI - PMC - PubMed
Xiang 2007 {published data only}
-
- Xiang PC, Ziang PC, Zhang X, Yang JN, Zhang EM, Gou WA, et al. The efficacy and safety of long term home noninvasive positive pressure ventilation in patients with stable severe chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2007;30(10):746-50. - PubMed
References to ongoing studies
Ankjaergaard {published data only}
Beth Israel Deaconess Medical Center {published data only}
-
- NCT04210050. Sleep ventilation for patients with advanced hypercapnic COPD [Implementation of a precision sleep ventilation (PSV) chronic respiratory failure management program for patients with advanced hypercapnic COPD]. clinicaltrials.gov/ct2/show/NCT04210050 (first received 24 December 2019).
Gonzales {published data only}
-
- NCT03890224. Respiratory support in chronic obstructive pulmonary disease (COPD) patients [Respiratory support in chronic obstructive pulmonary disease (COPD) patients after acute exacerbation with monitoring the quality of support]. clinicaltrials.gov/ct2/show/NCT03890224 (first received 26 March 2019).
Lamia {published data only}
-
- Lamia B, Cuvelier A, Benichou J, Muir JF. A multi-centre randomized controlled trial of domiciliary non-invasive ventilation vs long-term oxygen therapy in survivors of acute hypercapnic respiratory failure due to COPD. Non-invasive ventilation in obstructive lung disease (NIVOLD) study.. Revue des maladies respiratoires 2012;29(9):1141-8. - PubMed
-
- NCT03221101. Home non invasive ventilation for COPD patients (NIVOLD) [Home non invasive ventilation versus long term oxygen therapy alone in COPD survivors after acute hypercapnic respiratory failure. A French multicenter randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03221101 (first received 18 July 2017).
Pepin {published data only}
-
- NCT03584269. Innovation in NOn invasive ventilation in COPD patients treated by long term oxygen therapy (INOV-LTOT) [Non invasive ventilation and nocturnal alveolar hypoventilation in patients with chronic obstructive pulmonary disease (COPD) treated by long term oxygen therapy at home]. clinicaltrials.gov/show/nct03584269 (first received 12 July 2018).
Wijkstra {published data only}
-
- NCT03053973. The effects of nocturnal non-invasive ventilation in stable COPD (RECAPTURE) [Nocturnal non-invasive ventilation in COPD patients with stable hypercapnic respiratory failure: why and in which patient might this be effective?]. clinicaltrials.gov/show/nct03053973 (first received 15 February 2017).
Additional references
Ambrosino 1990
-
- Ambrosino N, Montagna T, Nava S, Negri A, Brega S, Fracchia C, et al. Short term effect of intermittent negative pressure ventilation in COPD patients with respiratory failure. European Respiratory Journal 1990;3:502-8. - PubMed
Bestall 1999
Chest 1999
Crimi 2016
Donaldson 2002
Dreher 2010
-
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010;65(4):303-8. - PubMed
Dretzke 2016
-
- Dretzke J, Moore D, Dave C, Mukherjee R, Price MJ, Bayliss S, et al. The effect of domiciliary noninvasive ventilation on clinical outcomes in stable and recently hospitalised patients with COPD: a systematic review and meta-analysis. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:2269-86. [DOI: 10.2147/COPD.S104238] - DOI - PMC - PubMed
Elliott 1991
-
- Elliott MW, Mulvey DA, Moxham J, Green M, Branthwaite MA. Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. European Respiratory Journal 1991;4(9):1044-52. - PubMed
Ergan 2019
-
- Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society Guideline on Long-term Home Non-Invasive Ventilation for Management of Chronic Obstructive Pulmonary Disease. European Respiratory Journal 2019;57:1901003. [DOI: 10.1183/13993003.01003-2019] - DOI - PubMed
GOLD 2021
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD 2021. Global Initiative for Chronic Obstructive Lung Disease (GOLD). goldcopd.org/2021-gold-reports/ 2021.
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading quality of evidence and strength of recommendations. GRADE Working Group, October 2013.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 1 July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hurst 2010
Jones 1991
-
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85(Suppl B):25-31; discussion 33-7. - PubMed
Macrea 2020
-
- Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM 3rd. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine 2020;202(4):e74-e87. [DOI: 10.1164/rccm.202006-2382ST] - DOI - PMC - PubMed
Magnet 2017
Mahler 1984
-
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85(6):751-8. - PubMed
Osadnik 2017
-
- Osadnik CR, Tee VS, Carson‐Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non‐invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD004104. [DOI: 10.1002/14651858.CD004104.pub4] - DOI - PMC - PubMed
Ottenheijm 2008
Puhan 2011
Quanjer 1993
-
- Quanjer PhH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Lung volumes and forced ventilatory flows. European Respiratory Journal 1993/03/01;6(Suppl 16):5. - PubMed
Raveling 2020
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rochester 1985
-
- Rochester DF, Braun NM. Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1985;132(1):42-7. - PubMed
Rodway 2010
Rossi 2000
-
- Rossi A, Hill NS. Non invasive ventilation has (not) been shown to be ineffective in stable COPD. American Journal of Respiratory and Critical Care Medicine 2000;161:688-91. - PubMed
RStudio 2020 [Computer program]
-
- RStudio: Integrated Development Environment for R.. RStudio Team . Boston, MA: RStudio, PBC, 2020.
Saatci 2004
Struik 2013
-
- Struik FM, Kerstjens HA, Bladder G, Sprooten R, Zijnen M, Asin J, et al. The Severe Respiratory Insufficiency Questionnaire scored best in the assessment of health-related quality of life in chronic obstructive pulmonary disease. Journal of Clinical Epidemiology 2013;66(10):1166-74. [DOI: 10.1016/j.jclinepi.2013.04.013] - DOI - PubMed
Struik 2014
Terzikhan 2016
van Dijk 2020
Welling 2015
-
- Welling J B, Hartman JE, Ten Hacken NH, Klooster K, Slebos DJ. The minimal important difference for the St George's Respiratory Questionnaire in patients with severe COPD. European Respiratory Journal 2015;46(6):1598-604. - PubMed
WHO 2020
-
- World Health Organization - Factsheet Chronic Obstructive Pulmonary Disease. www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-d... (accessed prior to 12 February 2021).
Wilson 2020
-
- Wilson ME, Dobler CC, Morrow AS, Beuschel B, Alsawas M, Benkhadra R, et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Journal of the American Medical Association 2020;323(5):455-65. [DOI: 10.1001/jama.2019.22343] - DOI - PMC - PubMed
Windisch 2003
-
- Windisch W, Freidel K, Schucher B, Baumann H, Wiebel M, Matthys H, et al. The Severe Respiratory Insufficiency (SRI) Questionnaire: a specific measure of health-related quality of life in patients receiving home mechanical ventilation. Journal of Clinical Epidemiology 2003;56:752-9. [DOI: 10.1016/s0895-4356(03)00088-x] - DOI - PubMed
Windisch 2008
-
- Windisch W, Budweiser S, Heinemann F, Pfeifer M, Rzehak P. The Severe Respiratory Insufficiency Questionnaire was valid for COPD patients with severe chronic respiratory failure. Journal of Clinical Epidemiology 2008;61(8):848-53. - PubMed
Windisch 2009
Windisch 2015
References to other published versions of this review
Struik 2013
-
- Struik FM, Lacasse Y, Goldstein R, Kerstjens HA, Wijkstra PJ. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease (Review). Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD002878. [DOI: 10.1002/14651858.CD002878.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

