Pharmacological Interventions for the Management of Cancer-Related Fatigue Among Cancer Survivors: Systematic Review and Meta-Analysis
- PMID: 34369188
- PMCID: PMC8358505
- DOI: 10.1177/15347354211038008
Pharmacological Interventions for the Management of Cancer-Related Fatigue Among Cancer Survivors: Systematic Review and Meta-Analysis
Abstract
Objective: Current guidelines have different recommendations on applying pharmacological interventions for managing cancer-related fatigue (CRF) among cancer survivors. This systematic review aims to synthesize clinical evidence on pharmacological interventions for managing CRF.
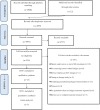
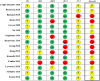
Methods: Five databases were searched for potential randomized controlled trials (RCTs) from their inception until October 2020. RCTs assessing the effect of pharmacological treatments for CRF among cancer survivors were considered eligible. Clinical significance was determined by comparing the estimated effect with that of minimal important difference (MID). The risk of bias of each included RCT was appraised using the Cochrane risk of bias tool for randomized trials 2. Data were synthesized using random-effect pairwise meta-analyses.
Results: A total of 15 RCTs (1238 participants) were included. The majority presented some concerns of bias arising from the randomization process and selection of the reported results. Meta-analysis showed that psychostimulant and wakefulness agents had statistically significant while clinically insignificant effects on the treatment of CRF (pooled weighted mean difference [WMD]: 2.8, 95% confidence interval [CI]: 0.2-5.4, I2: 0%, 3 RCTs, MID: 3.0-6.0). Three natural products, including Renshen Yangrong Tang (mean difference [MD]: -16.1, 95% CI: -8.9 to -23.3, MID: -17.3 to -11.4), Tualang honey (MD: 11.2, 95% CI: 7.1-15.3, MID: 3.0-6.0), and Shenmai injection plus Peptisorb (MD: -1.6, 95% CI: -2.1 to -1.1, MID: -1.1 to -0.8) demonstrated statistically and clinically significant effect in reducing CRF.
Conclusions: Existing evidence showed promising effects of 3 natural products in reducing CRF among cancer survivors. The results from this study need to be further confirmed with well-designed and adequately powered RCTs that use validated instruments for the measurement of CRF.
Keywords: cancer survivors; drug therapy; fatigue; meta-analysis; systematic review.
Conflict of interest statement
Figures

Similar articles
-
Meta-Analysis of Randomized Controlled Trials of Acupuncture for Cancer-Related Fatigue.Integr Cancer Ther. 2014 May;13(3):193-200. doi: 10.1177/1534735413510024. Epub 2013 Nov 25. Integr Cancer Ther. 2014. PMID: 24282102
-
Interventions for fatigue in inflammatory bowel disease.Cochrane Database Syst Rev. 2020 Apr 16;4(4):CD012005. doi: 10.1002/14651858.CD012005.pub2. Cochrane Database Syst Rev. 2020. PMID: 32297974 Free PMC article.
-
The Effects of Acupuncture on Cancer-Related Fatigue: Updated Systematic Review and Meta-Analysis.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420949679. doi: 10.1177/1534735420949679. Integr Cancer Ther. 2020. PMID: 32996339 Free PMC article.
-
The effects of Tai Chi on quality of life of cancer survivors: a systematic review and meta-analysis.Support Care Cancer. 2019 Oct;27(10):3701-3716. doi: 10.1007/s00520-019-04911-0. Epub 2019 Jun 24. Support Care Cancer. 2019. PMID: 31236699
-
Herbal medicine on cancer-related fatigue of lung cancer survivors: Protocol for a systematic review.Medicine (Baltimore). 2020 Jan;99(5):e18968. doi: 10.1097/MD.0000000000018968. Medicine (Baltimore). 2020. PMID: 32000424 Free PMC article.
Cited by
-
Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue.Cancers (Basel). 2022 Dec 23;15(1):91. doi: 10.3390/cancers15010091. Cancers (Basel). 2022. PMID: 36612088 Free PMC article. Review.
-
Effects of Managing Cancer and Living Meaningfully on Cancer-Related Fatigue and Cytokine Levels in Gastrointestinal Cancer Patients.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231172511. doi: 10.1177/15347354231172511. Integr Cancer Ther. 2023. PMID: 37249000 Free PMC article. Clinical Trial.
-
State of Rehabilitation Research in the Head and Neck Cancer Population: Functional Impact vs. Impairment-Focused Outcomes.Curr Oncol Rep. 2022 Apr;24(4):517-532. doi: 10.1007/s11912-022-01227-x. Epub 2022 Feb 19. Curr Oncol Rep. 2022. PMID: 35182293 Review.
-
Management of Fatigue in Patients with Advanced Cancer.Curr Treat Options Oncol. 2023 Feb;24(2):93-107. doi: 10.1007/s11864-022-01045-0. Epub 2023 Jan 19. Curr Treat Options Oncol. 2023. PMID: 36656503 Free PMC article. Review.
-
Chronic Fatigue in Cancer Survivorship: Psychiatry Versus Oncology or Psychiatry with Oncology?Curr Oncol Rep. 2025 Jul;27(7):883-905. doi: 10.1007/s11912-025-01697-9. Epub 2025 Jun 11. Curr Oncol Rep. 2025. PMID: 40498265 Free PMC article. Review.
References
-
- Pearson EJM, Morris ME, di Stefano M, McKinstry CE. Interventions for cancer-related fatigue: a scoping review. Eur J Cancer Care. 2018;27(1). doi:10.1111/ecc.12516. - PubMed
-
- NCCN Guidelines Version 2. Cancer-related fatigue. www.nccn.org. Accessed May 4, 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

