Statins significantly repress rotavirus replication through downregulation of cholesterol synthesis
- PMID: 34369301
- PMCID: PMC8354672
- DOI: 10.1080/19490976.2021.1955643
Statins significantly repress rotavirus replication through downregulation of cholesterol synthesis
Retraction in
-
Statement of Retraction: Statins significantly repress rotavirus replication through downregulation of cholesterol synthesis.Gut Microbes. 2022 Jan-Dec;14(1):2151146. doi: 10.1080/19490976.2022.2151146. Gut Microbes. 2022. PMID: 36420527 Free PMC article. No abstract available.
Abstract
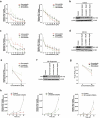
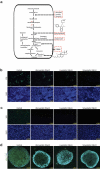
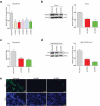
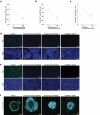
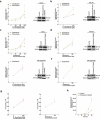
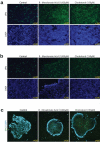
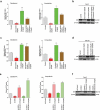
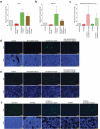
Rotavirus is the most common cause of severe diarrhea among infants and young children and is responsible for more than 200,000 pediatric deaths per year. There is currently no pharmacological treatment for rotavirus infection in clinical activity. Although cholesterol synthesis has been proven to play a key role in the infections of multiple viruses, little is known about the relationship between cholesterol biosynthesis and rotavirus replication. The models of rotavirus infected two cell lines and a human small intestinal organoid were used. We investigated the effects of cholesterol biosynthesis, including inhibition, enhancement, and their combinations on rotavirus replication on these models. The knockdown of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) was built by small hairpin RNAs in Caco2 cells. In all these models, inhibition of cholesterol synthesis by statins or HMGCR knockdown had a significant inhibitory effect on rotavirus replication. The result was further confirmed by the other inhibitors: 6-fluoromevalonate, Zaragozic acid A and U18666A, in the cholesterol biosynthesis pathway. Conversely, enhancement of cholesterol production increased rotavirus replication, suggesting that cholesterol homeostasis is relevant for rotavirus replication. The effects of all these compounds toward rotavirus were further confirmed with a clinical rotavirus isolate. We concluded that rotavirus replication is dependent on cholesterol biosynthesis. To be specific, inhibition of cholesterol synthesis can downregulate rotavirus replication; on the contrary, rotavirus replication is upregulated. Statin treatment is potentially an effective novel clinical anti-rotavirus strategy.
Keywords: Rotavirus infection; antiviral therapy; cholesterol synthesis; statins.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication.J Virol. 2011 Dec;85(23):12570-7. doi: 10.1128/JVI.05839-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957312 Free PMC article.
-
Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected Caco-2 cells.Arch Virol. 2015 Jun;160(6):1421-33. doi: 10.1007/s00705-015-2404-4. Epub 2015 Apr 1. Arch Virol. 2015. PMID: 25877820
-
Antiviral activities of resveratrol against rotavirus in vitro and in vivo.Phytomedicine. 2020 Oct;77:153230. doi: 10.1016/j.phymed.2020.153230. Epub 2020 Apr 18. Phytomedicine. 2020. PMID: 32682225
-
Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences.Clin Pharmacokinet. 1997 May;32(5):403-25. doi: 10.2165/00003088-199732050-00005. Clin Pharmacokinet. 1997. PMID: 9160173 Review.
-
Perspectives of the non-statin hypolipidemic agents.Pharmacol Ther. 2010 Jul;127(1):19-40. doi: 10.1016/j.pharmthera.2010.03.007. Epub 2010 Apr 24. Pharmacol Ther. 2010. PMID: 20420853 Review.
Cited by
-
Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in vitro.Front Microbiol. 2022 Mar 18;13:721103. doi: 10.3389/fmicb.2022.721103. eCollection 2022. Front Microbiol. 2022. PMID: 35369500 Free PMC article.
-
Host Cell Response to Rotavirus Infection with Emphasis on Virus-Glycan Interactions, Cholesterol Metabolism, and Innate Immunity.Viruses. 2023 Jun 21;15(7):1406. doi: 10.3390/v15071406. Viruses. 2023. PMID: 37515094 Free PMC article.
-
Piscine Vitamin D Receptors Vdra/Vdrb in the Absence of Vitamin D Are Utilized by Grass Carp Reovirus for Promoting Viral Replication.Microbiol Spectr. 2023 Aug 17;11(4):e0128723. doi: 10.1128/spectrum.01287-23. Epub 2023 Jul 19. Microbiol Spectr. 2023. PMID: 37466438 Free PMC article.
-
Advances in the development of antivirals for rotavirus infection.Front Immunol. 2023 Mar 17;14:1041149. doi: 10.3389/fimmu.2023.1041149. eCollection 2023. Front Immunol. 2023. PMID: 37006293 Free PMC article. Review.
-
Aspects of Antiviral Strategies Based on Different Phototherapy Approaches: Hit by the Light.Pharmaceuticals (Basel). 2022 Jul 13;15(7):858. doi: 10.3390/ph15070858. Pharmaceuticals (Basel). 2022. PMID: 35890156 Free PMC article. Review.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–141. doi:10.1016/S1473-3099(11)70253-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
